Authors:
Carla Hasson, BS
Medical Student, USF Health Morsani College of Medicine, Tampa, Florida
Elizabeth Melzer, MD
Assistant Chief Hospitalist, Patient Safety, James A. Haley Veterans’ Hospital, Tampa, Florida
Citation:
Hasson C, Melzer E. Spontaneous bilateral internal jugular vein clots with 2 causes of hypercoagulability. Consultant. 2019;59(12):376-378.
A 37-year-old man presented with left-sided anterolateral neck pain for 1 day that had begun without preceding injury. He described the pain as dull and nonradiating, and he rated it 5 of 10 on the pain scale. He also noted a frontotemporal headache but denied experiencing any vision changes or neurologic symptoms.
History. His medical history was significant for essential thrombocythemia (ET) with JAK2 mutation on bone marrow biopsy, as well as thrombosis of the greater saphenous vein for which he had been started on enoxaparin (low-molecular-weight heparin for anticoagulation and bridge to warfarin) and warfarin (vitamin K antagonist anticoagulant) 2 weeks prior to presentation. He denied preceding periods of immobility and had no history of venous thromboembolism (VTE). His medications included hydroxyurea (for myeloproliferative disorder), enoxaparin, and warfarin. He reported having medication allergies to anagrelide and allopurinol. He denied a family history of hypercoagulability or VTE, and he had 2 healthy children. He was a lifetime nonsmoker and consumed 1 or 2 alcoholic mixed drinks daily. Review of systems was unremarkable aside from the history of present illness.
Physical examination. At presentation, the patient’s temperature was 37.1°C, blood pressure was 153/91 mm Hg, heart rate was 96 beats/min, and oxygen saturation was normal. No cervical swelling or lymphadenopathy was noted. The patient had full range of motion of the neck with tenderness to palpation of the anterolateral left neck. The remainder of the examination findings were normal except for a small ecchymosis on his abdomen at injection sites and tenderness to palpation of the right calf with positive Homan sign, indicating the possible presence of deep-vein thrombosis (DVT).
Diagnostic tests. Initial laboratory workup revealed the following values: white blood cell count, 5570/µL (reference range, 3900-990/µL); hemoglobin, 14.2 g/dL (reference range, 13-17 g/dL); hematocrit, 40.7% (reference range, 39%-49%); platelet count, 345 × 103/µL (patient’s baseline, 700-800 × 103/µL); prothrombin time, 10.1 seconds (reference range, 8.7-11.7 seconds); activated partial thromboplastin time, 34.6 seconds (reference range, 26.3-37.3 seconds); and international normalized ratio, 1.02 (reference range, 0.87-1.18). Results of a basic metabolic panel were normal. Results of liver function tests were normal except for mildly elevated transaminases (aspartate aminotransferase, 83 U/L [reference range, 16-43 U/L]; alanine aminotransferase, 51 U/L [reference range, 11-44 U/L]). The anti-factor Xa level was 0.4 IU/mL (where >1 IU/mL indicates the therapeutic range for enoxaparin).
Bilateral upper extremity Doppler venous ultrasonography showed acute DVTs of the bilateral internal jugular veins (Figure) and left subclavian vein. Findings of magnetic resonance venography of the brain were negative for cavernous sinus thrombosis.
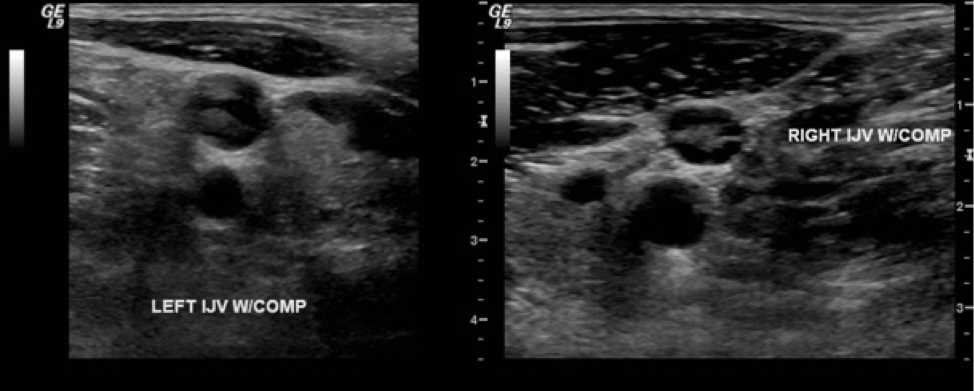
Figure. Left and right internal jugular DVTs on Doppler venous ultrasonography imaging.
The patient was started on a heparin drip, and his warfarin dose was increased. A hypercoagulability workup revealed a homozygous factor V Leiden mutation, the absence of a prothrombin G20210A mutation, normal protein C and S levels, and the absence of anticardiolipin antibodies. A consultant hematologist-oncologist recommended lifelong warfarin and indefinite anticoagulation.
Outcome of the case. The patient’s case was followed in the anticoagulation clinic, and he had no additional thrombosis events.
Discussion. ET is a myeloproliferative disorder characterized by megakaryocyte proliferation that leads to increased circulating platelets and hemorrhagic and thrombotic complications.1 High-risk ET is defined by age above 60 years or a thrombosis history.2 Commonly used front-line therapies for high-risk ET patients include hydroxyurea and α-interferon, with anagrelide as second-line therapy.3
Factor V Leiden heterozygosity occurs in 3% to 8% of the general US and European populations, with a higher prevalence among the white population.4 The prevalence of factor V Leiden in patients with ET is comparable, but the mutation increases the risk of recurrent VTE.5 It is a major contributor to thrombotic episodes in ET, while prothrombin G20210A and methylenetetrahydrofolate reductase (MTHFR) 677C>T and MTHFR 1298A>C mutations are not.6 Random factor V Leiden screening of the general population is not recommended; however, testing for the mutation should be performed in patients younger than 50 years with any VTE.7
Our patient was tested for additional thrombophilias because he wanted to know about risk factors for his children. The results did not change his management, because recurrent VTE necessitates lifelong anticoagulation therapy.
REFERENCES:
- Okoli S, Harrison C. Emerging treatments for essential thrombocythemia. J Blood Med. 2011;2:151-159.
- Tefferi A. Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87(3):285-293.
- Barbui T, Finazzi MC, Finazzi G. Front-line therapy in polycythemia vera and essential thrombocythemia. Blood Rev. 2012;26(5):205-211.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med. 2011;13(1):1-16.
- Ruggeri M, Gisslinger H, Tosetto A, et al. Factor V Leiden mutation carriership and venous thromboembolism in polycythemia vera and essential thrombocythemia. Am J Hematol. 2002;71(1):1-6.
- Trifa AP, Cucuianu A, Popp RA, et al. The relationship between factor V Leiden, prothrombin G20210A, and MTHFR mutations and the first major thrombotic episode in polycythemia vera and essential thrombocythemia. Ann Hematol. 2014;93(2):203-209.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med. 2001;3(2):139-148.


