Peer Reviewed
Focal Atypical Lymphoid Infiltrate With CD30 Positivity in a 36-Year-Old Man
Authors:
Alexandria B. Glass, DO
Resident Physician, Philadelphia College of Osteopathic Medicine (PCOM) Dermatology Residency, North Fulton Hospital, Roswell, Georgia
Christopher M. Buckley, DO
Faculty, PCOM Dermatology Residency, North Fulton Hospital, Roswell, Georgia
Dipti Anand, MD
Faculty, PCOM Dermatology Residency, North Fulton Hospital, Roswell, Georgia; SkinPath Solutions, Smyrna, Georgia
Evangelos G. Poulos, MD
Aurora Diagnostics Global Pathology, Miami Lakes, Florida
Marcus B. Goodman, DO
Program Director, PCOM Dermatology Residency, North Fulton Hospital, Roswell, Georgia
Citation:
Glass AB, Buckley CM, Anand D, Poulos EG, Goodman MB. Focal atypical lymphoid infiltrate with CD30 positivity in a 36-year-old man. Consultant. 2019;59(1):27-30
A 36-year-old man presented to the clinic for the evaluation of a new growth under his right arm. He stated that the growth had begun 1 to 2 months prior and had progressively increased in size.
History. The patient reported tenderness surrounding the lesion but denied recent fevers, chest pain, shortness of breath, gastrointestinal tract discomfort, rash, and edema. His medical history was significant only for hypertension, managed with losartan, and pertinent family medical history was noncontributory.
Physical examination. A single ulcerated, erythematous nodule was present posterolateral to the right axilla, with faint erythema surrounding the lesion (Figures 1 and 2).
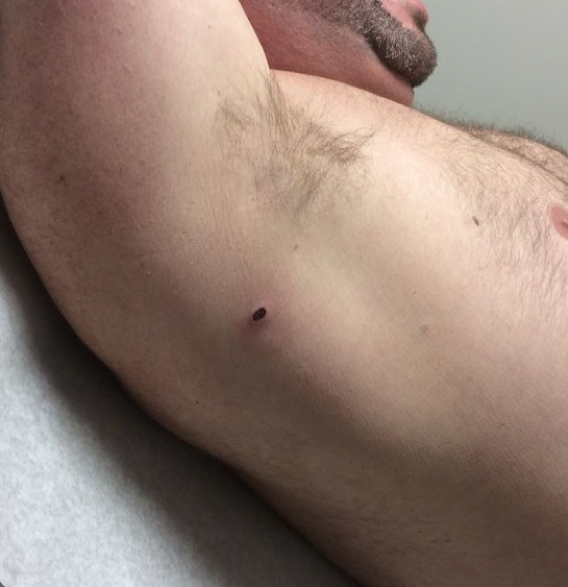
Figure 1. The lesion on initial presentation
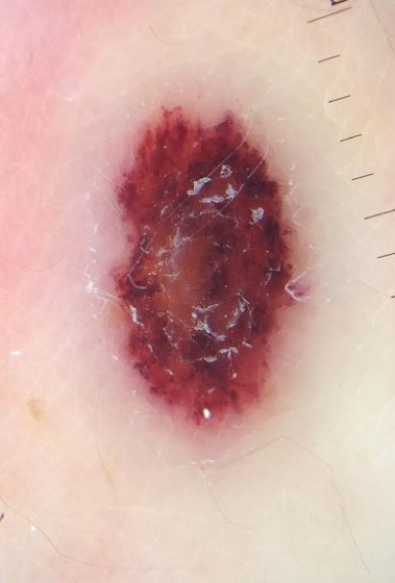
Figure 2. Dermoscopic view of the lesion.
A shave biopsy was performed and sent to dermatopathology for evaluation. The patient was prescribed oral doxycycline to treat the possibility of a localized cellulitis.
Diagnostic tests. Histology test results revealed an atypical T-cell lymphoid infiltrate with CD30 positivity of the dermis, extensive epidermal and dermal necrosis, hemorrhage, and fibrinopurulent exudate. The differential diagnosis included type C variant of lymphomatoid papulosis (LyP), primary cutaneous CD30-positive anaplastic large-cell lymphoma (PCALCL), and metastatic visceral/nodal T-cell lymphoma.
Due to the unusual nature of the lesion, the case was referred to the National Cancer Institute (NCI) for further review. The NCI report revealed similar findings of an atypical T-cell infiltrate with superficial ulceration and necrosis (Figure 3). Multiple immunostains were performed, which showed that the lymphoid cells were positive for CD3 and CD4 and negative for CD8. CD20 immunostaining highlighted some extremely rare B cells. CD30 immunostaining showed focal and variable positivity (Figure 4). Stains for varicella zoster virus (VZV) and herpes simplex virus (HSV) 1 and 2 were negative.
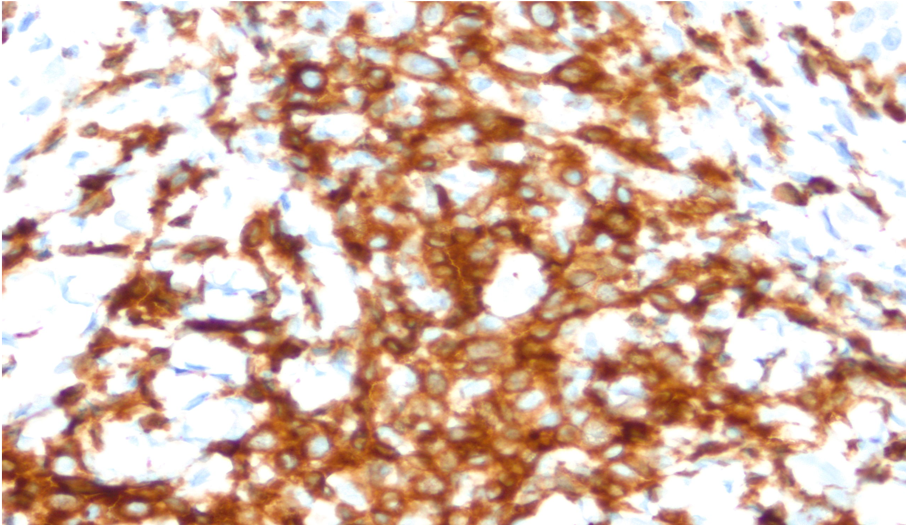
Figure 3. Histologic image of the patient’s lesion demonstrating focal atypical lymphoid infiltrate
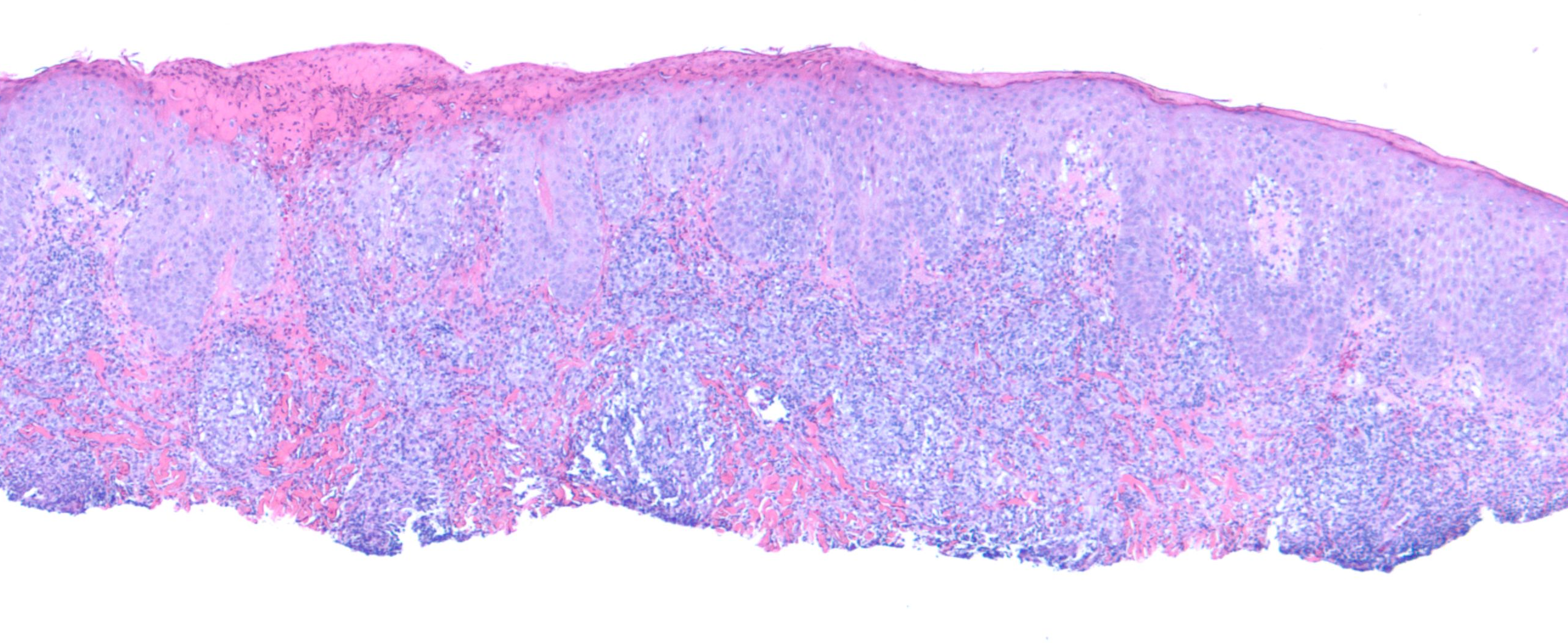
Figure 4. Pathology image showing CD30 positivity.
Due to these findings, the possibility of a primary cutaneous CD30 positive T-cell lymphoproliferative disorder could not be ruled out, and complete excision was recommended.
Outcome of the case. The lesion was excised in the office, with resultant clear margins found on pathology. The patient was then referred to an oncologist for further workup to rule out a systemic or node-based lymphoma. Findings of all additional testing, including full-body positron-emission tomography/computed tomography scans, were negative. After 2½ years, the patient had been doing well and was being followed up by an oncologist for routine monitoring.
Discussion. Cutaneous T-cell lymphoma (CTCL) encompasses various lymphomas derived from transformed T-lymphocytes, including mycosis fungoides (MF), CD30-positive T-cell lymphoproliferative disorders, and subcutaneous T-cell lymphoma. The CD30-positive T-cell lymphomas follow MF as the second most common category of CTCLs and account for 30% of all primary cutaneous lymphomas.
As the name implies, these disorders share the expression by atypical lymphocytes of CD30, a cytokine receptor that belongs to the tumor necrosis factor receptor family and is involved in the process of tumor cell growth. Disorders in this category are PCALCL, LyP, borderline disorders, transformed-stage MF, and systemic anaplastic large cell lymphoma (ALCL). Although they possess similar expression of CD30, these disorders can be very different in their clinical presentation and progression.
The findings in this patient’s case showed an atypical CD30-positive lymphoid infiltrate; however, the exact diagnosis was inconclusive. The differential diagnosis included PCALCL, LyP, borderline lesions, cutaneous metastasis from a nodal or systemic ALCL, CD30-positive transformed MF, rare types of CTCL that can occasionally show CD30 positivity, and reactive processes such as drug reactions, arthropod bites, and viral infections.1-3
PCALCL and LyP have many overlapping clinical and histological features, making the definitive diagnosis difficult, especially if the decision is based solely on histological findings. The clinical appearance of the lesion, in conjunction with histology tests, are used to determine a diagnosis and treatment. In patients where a distinction between PCALCL and LyP cannot be made, the working diagnosis term borderline case is used. After vigilant longitudinal clinical evaluation of the patient, a diagnosis of PCALCL or LyP usually can be achieved based on clinical behavior. Thus, careful clinical correlation is critical when managing a CD30-positive lymphoid infiltrate.3
PCALCL accounts for approximately 12% of all CTCLs. Most patients with PCALCL present with an ulcerated, rapidly growing solitary tumor or grouped nodules. They are most common in the 6th decade of life, are twice as common in men than in women, primarily affect adults, and tend to appear on the head, neck, and extremities. Histologically, the tumor is composed of large, anaplastic, pleomorphic lymphocytes with irregular nuclei and abundant cytoplasm arranged in sheets infiltrating the deep dermis and subcutaneous tissue. More than 75% of lymphocytes stain positive for CD30. They also commonly express a CD4+ T-cell phenotype, whereas less than 5% have a CD8+ T-cell phenotype.
Expression of IRF4 (interferon regulatory factor 4; also known as MUM1, multiple myeloma oncogene 1) is present in almost all cases, and there is variable loss of CD2, CD3, and/or CD5. If positivity is observed for anaplastic lymphoma kinase (ALK) or epithelial membrane antigen (EMA), then chromosomal translocation of t(2;5) is likely, and further workup for systemic or nodal ALCL should be performed.
Treatment for PCALCL includes excising the lesion in its entirety. If the lesion is too large to be excised or is excised with involved margins, radiotherapy should be performed in conjunction with excision.1,3,4 In 10% to 42% of cases, the lesion may partially or completely regress as in LyP; however, recurrences are common and treatment is necessary for remission.5,6 The prognosis is generally favorable, with a 5-year survival rate between 76% and 96%.7
On the other hand, LyP is a chronic, recurrent, self-healing, papulonodular skin disorder. It was first described by dermatologist Warren L. Macaulay, MD, in 1968 and can present very similarly to a PCALCL.8 There has been continuous debate as to whether LyP is a benign or malignant lymphoproliferative process. Due to its CD30 positivity, the presence of abnormal T cells, and its similarity to PCALCL, LyP is most commonly categorized as a low-grade variant of CTCL. The etiology of LyP is unknown, and multiple attempts to correlate a viral etiology, such as human T-cell lymphotropic virus type 1, Epstein-Barr virus (EBV), and HSV-1 and HSV-2, have been unsuccessful.9
The mean age of occurrence for LyP is between 35 to 45 years, but it can occur in any age group. It has a slight male predominance, with the male to female ratio being approximately 1.5 to 1.
There are 5 distinct histological subtypes (A-E) of LyP with different clinical presentations but all exhibiting CD30 positivity. Type A is the most common variant and presents as papulonodular lesions. Type B is uncommon and generally presents as an MF-type plaque that comes and goes. Type C presents as a solitary regressing nodule, resembling features of PCALCL. Type D and E represent more unusual variants of LyP. Type D appears as localized erythematous scaly lesions, resembling pagetoid reticulosis. Type E, the newest described variant, is the angioinvasive type that has an association with EBV, similar to extranodal nasal NK/T-cell lymphoma.1-4
LyP can persist for years to decades but is not associated with mortality.6,10 Nevertheless, a subgroup of patients with LyP develop a second lymphoid neoplasm either before, concurrently, or after the development of LyP. The “LyP-associated malignant lymphomas” are predominately MF, Hodgkin lymphoma, and cutaneous or systemic ALCL. The reported prevalence of developing these secondary lymphomas ranges from none to 62% of patients with LyP.10-14
Multiple treatment options such as psoralen–UV-A light therapy, low-dose methotrexate, and topical corticosteroids can help alleviate disseminated or stigmatizing lesions, but no treatment option alters the course of the disease. Therefore, the “wait and see” strategy is an appropriate first-line approach to these patients. Since the main concern is the development of a secondary lymphoma, patients with LyP should be monitored for life.1,2,4,6
Other less likely diagnoses included large-cell transformation MF. In any patient with CD30-positive lymphoid infiltrate and a history of MF, this diagnosis must be considered.15-17 Other non-neoplastic skin conditions can occur as part of a reactive activated T-cell response. CD30-positive lymphoid infiltrates have been described in drug reactions,18,19 atopic dermatitis,20 pityriasis lichenoides et varioliformis acuta,21 keratoacanthomas,22 and many infections including tuberculosis23 and multiple viruses. Associated viruses include verruca vulgaris caused by human papillomavirus,24 herpes viruses (such as HSV-1, HSV-2, and VZV),25 molluscum contagiosum virus,26 EBV,27 milker’s nodules caused by parapoxvirus,26,28 and HIV.29 Furthermore, arthropod assaults have caused CD30-positive lymphoid infiltrates, including scabies,30 tick bites,31 and spider bites.32 All of these non-neoplastic disorders should be ruled out prior to making the diagnosis of a CTCL.
Based on our patient’s history, clinical presentation, and histology findings, he likely had a PCALCL. Close monitoring of the patient is being performed; if he develops other lesions in the future, LyP type C may be a consideration. Our patient’s lesion was excised before adequate time could be given for regression. Regardless of the final diagnosis, this case demonstrated the importance of clinicopathological correlation in evaluating CD30-positive atypical lymphoid infiltrates.
- Kempf W. Cutaneous CD30-positive lymphoproliferative disorders. Surg Pathol Clin. 2014;7(2):203-228.
- Kempf W, Kerl K, Mitteldorf C. Cutaneous CD30-positive T-cell lymphoproliferative disorders—clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37(1):24-29.
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. Philadelphia, PA: Elsevier; 2012:2017-2036.
- Kempf W. A new era for cutaneous CD30-positive T-cell lymphoproliferative disorders. Semin Diagn Pathol. 2017;34(1):22-35.
- Vergier B, Beylot-Barry M, Pulford K, et al. Statistical evaluation of diagnostic and prognostic features of CD30+ cutaneous lymphoproliferative disorders: a clinicopathologic study of 65 cases. Am J Surg Pathol. 1998;22(10):1192-1202.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118(15):4024-4035.
- Benner MF, Willemze R. Applicability and prognostic value of the new TNM classification system in 135 patients with primary cutaneous anaplastic large cell lymphoma. Arch Dermatol. 2009;145(12):1399-1404.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign—histologically malignant. Arch Dermatol. 1968;97(1):23-30.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: A clue to the pathophysiology of clinical regression. Blood. 1999;94(9):3077-3083.
- Bekkenk MW, Geelen FAMJ, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95(12):3653-3661.
- Thomsen K, Wantzin GL. Lymphomatoid papulosis: a follow-up study of 30 patients. J Am Acad Dermatol. 1987;17(4):632-636.
- Kunishige JH, McDonald H, Alvarez G, Johnson M, Prieto V, Duvic M. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34(5):576-581.
- Christensen HK, Thomsen K, Vejlsgaard GL. Lymphomatoid papulosis: a follow-up study of 41 patients. Semin Dermatol. 1994;13(3):197-201.
- Gan EY, Tang MBY, Tan SH. Lymphomatoid papulosis: is a second lymphoma commoner among East Asians? Clin Exp Dermatol. 2012;37(2):118-121.
- Salhany KE, Cousar JB, Greer JP, Casey TT, Fields JP, Collins RD. Transformation of cutaneous T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol. 1988;132(2):265-277.
- Diamandidou E, Colome-Grimmer M, Fayad L, Duvic M, Kurzrock R. Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood. 1998;92(4):1150-1159.
- Benner MF, Jansen PM, Vermeer MH, Willemze R. Prognostic factors in transformed mycosis fungoides: a retrospective analysis of 100 cases. Blood. 2012;119(7):1643-1649.
- Chen Y-C, Wu Y-H. Linear folliculotropic CD30-positive lymphomatoid drug reaction. Am J Dermatopathol. 2017;39(5):e62-e65.
- Saeed SA, Bazza M, Zaman M, Ryatt KS. Cefuroxime induced lymphomatoid hypersensitivity reaction. Postgrad Med J. 2000;76(899):577-579.
- Piletta PA, Wirth S, Hommel L, Saurat JH, Hauser C. Circulating skin-homing T cells in atopic dermatitis: selective up-regulation of HLA-DR, interleukin-2R, and CD30 and decrease after combined UV-A and UV-B phototherapy. Arch Dermatol. 1996;132(10):1171-1176.
- Kempf W, Kazakov DV, Palmedo G, Fraitag S, Schaerer L, Kutzner H. Pityriasis lichenoides et varioliformis acuta with numerous CD30+ cells: a variant mimicking lymphomatoid papulosis and other cutaneous lymphomas. A clinicopathologic, immunohistochemical, and molecular biological study of 13 cases. Am J Surg Pathol. 2012;36(7):1021-1029.
- Fernandez-Flores A. CD30+ cell population in common keratoacanthomas: a study of 21 cases. Rom J Morphol Embryol. 2008;49(2):159-162.
- Massi D, Trotta M, Franchi A, Pimpinelli N, Santucci M. Atypical CD30+ cutaneous lymphoid proliferation in a patient with tuberculosis infection. Am J Dermatopathol. 2004;26(3):234-236.
- Cesinaro AM, Maiorana A. Verruca vulgaris with CD30-positive lymphoid infiltrate: a case report. Am J Dermatopathol. 2002;24(3):260-263.
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30(1):50-58.
- Werner B, Massone C, Kerl H, Cerroni L. Large CD30-positive cells in benign, atypical lymphoid infiltrates of the skin. J Cutan Pathol. 2008;35(12):1100-1107.
- Chai C, White WL, Shea CR, Prieto VG. Epstein Barr virus-associated lymphoproliferative-disorders primarily involving the skin. J Cutan Pathol. 1999;26(5):242-247.
- Rose C, Starostik P, Bröcker E-B. Infection with parapoxvirus induces CD30-positive cutaneous infiltrates in humans. J Cutan Pathol. 1999;26(10):520-522.
- Smith KJ, Barrett TL, Neafie R, et al. Is CD30 (Ki-1) immunostaining in cutaneous eruptions useful as a marker of Th1 to Th2 cytokine switching and/or as a marker of advanced HIV-1 disease? Br J Dermatol. 1998;138(5):774-779.
- Gallardo F, Barranco C, Toll A, Pujol RM. CD30 antigen expression in cutaneous inflammatory infiltrates of scabies: a dynamic immunophenotypic pattern that should be distinguished from lymphomatoid papulosis. J Cutan Pathol. 2002;29(6):368-373.
- Hwong H, Jones D, Prieto VG, Schulz C, Duvic M. Persistent atypical lymphocytic hyperplasia following tick bite in a child: report of a case and review of the literature. Pediatr Dermatol. 2001;18(6):481-484.
- Cepeda LT, Pieretti M, Chapman SF, Horenstein MG. CD30-positive atypical lymphoid cells in common non-neoplastic cutaneous infiltrates rich in neutrophils and eosinophils. Am J Surg Pathol. 2003;27(7):912-918.


