Chronic Obstructive Pulmonary Disease: Bronchodilators and Reversibility of Airflow Obstruction
ABSTRACT: A central feature of chronic obstructive pulmonary disease (COPD) is airflow obstruction that is not fully reversible, defined as a postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of < 0.70. Although airflow obstruction in COPD is not fully reversible, patients can still exhibit clinically significant bronchodilator reversibility (or responsiveness), based on changes from prebronchodilator values in FEV1 and/or FVC. The FVC component is particularly important in patients with more severe COPD who have greater hyperinflation, as these patients often show clinically meaningful improvement in FVC without a significant FEV1 response after bronchodilator treatment. Confusion exists surrounding the concept of bronchodilator reversibility because of the lack of a standardized definition of and methodologies for assessing reversibility, inherent day-to-day variability in bronchodilator response, and similarities with asthma in some cases. This article reviews the role of spirometry in COPD diagnosis and ongoing monitoring, definitions of bronchodilator reversibility, and clinical relevance of bronchodilator responsiveness in patients with COPD.
_____________________________________________________________________________________________________________________________
Chronic airflow obstruction is a central feature of chronic obstructive pulmonary disease (COPD).1 Often, patients with COPD have a significant response to bronchodilator treatment,2-5 but the disease is not fully reversible.1 Airflow limitation that is not fully reversible is defined as a postbronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of < 0.70.1 A common misconception is that patients who exhibit a significant response to bronchodilators but are not fully reversible more likely have asthma than COPD. However, bronchodilator responsiveness is not sensitive or specific enough to differentiate asthma from COPD.6 According to current COPD management guidelines, measurements of postbronchodilator FEV1/FVC and FEV1 are recommended for COPD diagnosis and assessment of disease severity, respectively, but the degree of postbronchodilator response (eg, change in FEV1) is not recommended for differentiation of COPD from asthma.1
DIFFERENTIATING BETWEEN COPD AND ASTHMA
Characteristic COPD symptoms include dyspnea, chronic cough, and sputum production.1 These symptoms also may be present in patients with asthma, albeit with greater variability.7 Several clinical features can help distinguish COPD from asthma (Table 1),1 although these distinguishing features are not absolute. Fixed airflow obstruction is a defining feature of COPD, but airway remodeling occurs often in asthma and may lead to a fixed component of airflow obstruction in some asthma patients.7 In particular, patients with longstanding asthma may demonstrate irreversible airway obstruction,1 and some patients with asthma may have a pseudo-emphysematous pattern.8 While atopy is very common in asthma,1 some COPD patients also have atopy.9 Although smoking is the most common cause of COPD,1 10% to 15% of patients with COPD have never smoked.10 While inflammation in asthma is dominated by eosinophils,11 patients with severe asthma may have predominantly neutrophils in their sputum,11,12 a characteristic more typical of COPD.11 Conversely, up-regulation of sputum eosinophils may occur in patients with COPD during exacerbations.11,13 It also is important to recognize that COPD and asthma can coexist in some patients.14

Some typical physiologic characteristics of COPD may help in further distinguishing COPD from asthma.15 The diffusing capacity of the lung for carbon monoxide typically is decreased in patients with the emphysematous phenotype of COPD but is normal or increased in patients with asthma.15,16 In addition, patients with COPD tend to have more severe hyperinflation at rest that increases with exertion, while resting hyperinflation usually is present in patients with asthma only during exacerbations.15 Patients with COPD with an emphysematous component have decreased lung elastic recoil.15 Lung elastic recoil generally is normal in patients with asthma,15 although it may be decreased in patients with asthma who have fixed airflow obstruction8 or transiently decreased during an acute asthma exacerbation.17 Thus, several clinical and physiologic features may factor into a diagnosis of COPD or asthma.
SPIROMETRY AND ASSESSING COPD
Spirometry is essential for confirming a diagnosis of COPD,1 which should be suspected in current or ex-smokers aged ≥ 40 years with dyspnea, chronic cough, or sputum,1 particularly those with a smoking history of ≥ 20 pack-years.18 In addition to a reduced FEV1/FVC ratio, patients with COPD generally have decreases in FEV1 and FVC, with greater declines in both measures as disease severity worsens.1
Performing conventional spirometry can be expensive and time-consuming, and it requires training and expertise.19 Handheld spirometers are an inexpensive, convenient, and practical alternative in the primary care setting.20 They are also accurate,21 with good correlation in FEV1 measurements between handheld spirometers and full spirometry.19 If findings from handheld or smaller pocket spirometers are abnormal, patients could be referred to a hospital-based pulmonary function laboratory or a specialist for more rigorous tests with conventional spirometry instruments.
If interpreted correctly, spirometry results can reveal important information about the patient’s disease. An FEV1/FVC ratio of < 0.70 that remains < 0.70 after bronchodilator administration (eg, albuterol 90 µg × 2 to 4 inhalations) confirms airflow obstruction that is not fully reversible, suggesting COPD.1 In patients who receive a diagnosis of COPD, spirometry findings also are used to assess disease severity and progression of the disease. Patients with FEV1 ≥ 80% predicted, FEV1 50% to < 80% predicted, 30% to < 50% predicted, or < 30% predicted are classified as having mild (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage I), moderate (GOLD stage II), severe (GOLD stage III), or very severe (GOLD stage IV) COPD, respectively.1 In addition, spirometry findings are used to assess individual patients’ changes in pulmonary function over time and after bronchodilator administration22; several criteria have been established for determining a clinically meaningful bronchodilator response (Table 2).22-25

ASSESSING BRONCHODILATOR REVERSIBILITY (RESPONSIVENESS)
Confusion exists surrounding the concept of bronchodilator reversibility or responsiveness for several reasons, including the lack of standardized definitions or methodologies for determining bronchodilator reversibility. The American Thoracic Society/European Respiratory Society (ATS/ERS) criteria for bronchodilator reversibility22 are the most current definition and include an FVC component, defining reversibility using FEV1, FVC, or both.22 The FVC component of the ATS/ERS definition is often overlooked, and although FVC results are more difficult to obtain, volume changes based on FVC may have a better correlation with improvements in exercise tolerance than measures of flow based on FEV1 and hence may be more clinically important.26 Moreover, some patients who do not demonstrate bronchodilator reversibility based on FEV1 do show a response based on FVC,5,27,28 particularly those with more severe COPD5 and greater hyperinflation.28
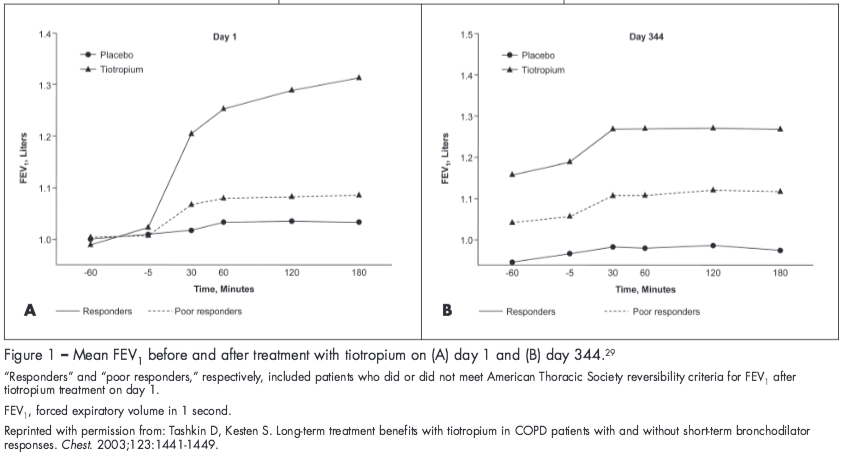
It should be noted that a patient’s acute response to bronchodilator treatment cannot be used to predict a long-term response to maintenance bronchodilator therapy.3,29 In a study by Tashkin and Kesten,29 tiotropium resulted in significant improvements in FEV1 from baseline compared with placebo after nearly a year of treatment, even in patients who did not show an initial short-term response to tiotropium (Figure 1). Similarly, responsiveness to salmeterol treatment was demonstrated at day 84 in patients classified as either “reversible” or “nonreversible” based on the ATS criteria for FEV1 after albuterol administration at baseline.3 However, in both studies, the magnitude of response to bronchodilator treatment was greater in patients classified as “reversible” or “a responder” at baseline.3,29
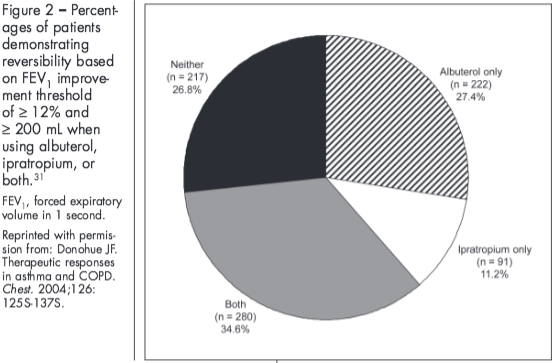
Further contributing to the confusion surrounding bronchodilator reversibility, bronchodilator response can vary based on the definition of reversibility used,30 the bronchodilator used,31 and day-to-day variability in bronchodilator response.30,32 In the Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) study, patients’ reversibility status (“responsive” or “poorly responsive”) varied depending on the criterion used.5 Similarly, findings from another study showed differences in the percentage of patients with COPD who exhibited reversibility after maximal bronchodilation (al-buterol 400 µg followed 30 minutes later by ipratropium 80 µg) depending on the criterion used, with 42% meeting the ATS criteria for FEV1 and 23% meeting the ERS criterion for % predicted FEV1.30 Bronchodilator response also varies based on the bronchodilator used.31 Differences in the percentage of patients exhibiting reversibility have been shown when using ipratropium alone, albuterol alone, or both (Figure 2).31
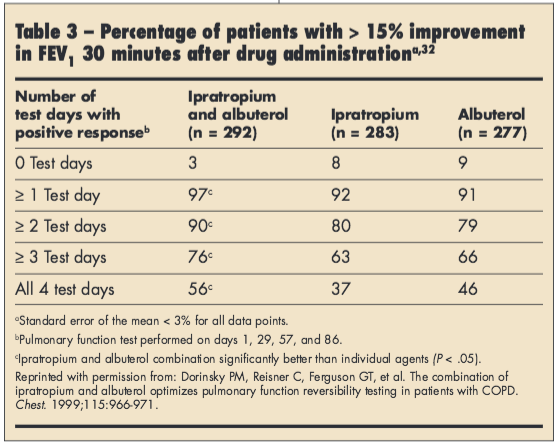
In addition, there is inherent day-to-day variability in bronchodilator response. In a study of 852 patients with moderate to severe COPD, the percentage of patients exhibiting significant (≥ 15% FEV1 improvement) reversibility to albuterol, ipratropium, or both varied over 4 separate days, with > 90% of patients demonstrating significant reversibility on at least 1 occasion (Table 3).32 In another study, 52% of patients changed reversibility status to albuterol and ipratropium over the 3 study visits based on the combined ATS criteria.30 However, despite variability in bronchodilator response, many patients with COPD do exhibit significant bronchodilator reversibility.
STUDIES SUPPORTING BRONCHODILATOR RESPONSIVENESS IN COPD
In a post hoc analysis of 5756 patients in the UPLIFT study, more than half of the patients exhibited bronchodilator responsiveness after maximal or near-maximal bronchodilation with ipratropium followed by albuterol treatment based on the combined ATS criteria for FEV1 improvement of ≥ 12% and ≥ 200 mL.5 Similarly, in 2 studies of 1109 patients with moderate to very severe COPD, a large percentage of patients demonstrated meaningful improvements in FEV1 based on the combined ATS criteria after administration of formoterol-containing treatment on the day of randomization (51% to 54%) and at the end of treatment (57% to 76%).4 Notably, at the screening visit, only 34% to 39% of patients exhibited reversibility to albuterol based on the combined ATS criteria for FEV1.4 These findings suggest that patients’ bronchodilator responsiveness may be underestimated if based on al-buterol reversibility testing alone.
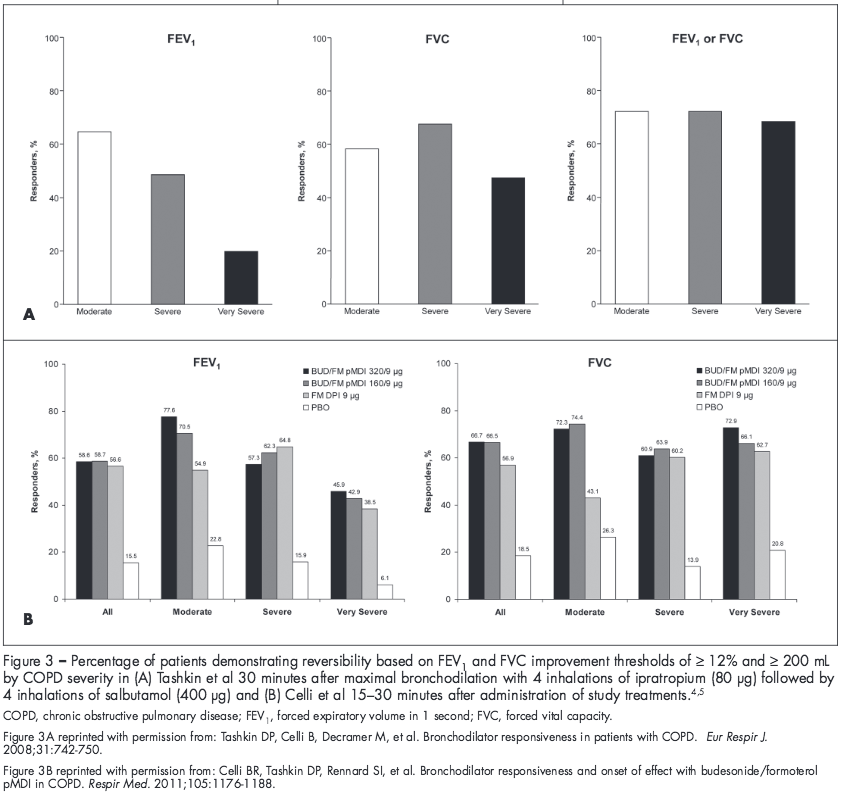
A patient’s response to a bronchodilator can be influenced by disease severity, depending on the criterion used. Studies have shown that bronchodilator response based on FEV1 may vary by disease severity, but response based on FVC remains more consistent across severity groups (Figure 3).4,5 In both the UPLIFT study analysis and the study conducted by Celli and colleagues, the percentage of patients demonstrating a bronchodilator response (FEV1 improvement ≥ 12% and ≥ 200 mL) progressively decreased with increasing COPD severity.4,5 In contrast, the percentage of patients exhibiting a bronchodilator response based on a FVC improvement of ≥ 12% and ≥ 200 mL generally was consistent across disease severities.4,5
BRONCHODILATORS FOR TREATING COPD
Bronchodilators are the mainstay of treatment for COPD.1 Combining different classes of bronchodilators may result in greater efficacy and a decreased risk of side effects compared with increasing the dose of a single bronchodilator.1 There also may be clinical benefits of adding an inhaled corticosteroid to long-acting bronchodilator therapy, in addition to reducing exacerbations.1 Data from the 2 studies of 1109 patients with moderate to very severe COPD showed that patients receiving budesonide/formoterol pressurized metered-dose inhaler (pMDI) generally had numerically greater mean improvements in 1-hour postdose FEV1 and FVC versus formoterol alone on the day of randomization.4 For the whole population (including all patients with moderate to very severe COPD), mean improvements in 1-hour postdose FEV1 and FVC on the day of randomization were 220 to 230 mL and 400 to 410 mL, respectively, in the budesonide/formoterol pMDI groups compared with 160 mL and 350 mL, respectively, in the formoterol group.4
CONCLUSIONS
COPD is characterized by airflow obstruction that is not fully reversible; however, confusion exists surrounding the concept of reversibility. Although the disease is not fully reversible, patients with COPD often exhibit a clinically significant bronchodilator response, as defined by several different published criteria for FEV1 and/or FVC improvement (eg, American College of Chest Physicians, ATS/ERS, ATS). In particular, patients with more severe COPD show benefits of reduced air trapping and hyperinflation after bronchodilator treatment, as measured by FVC. Bronchodilators are central to COPD management, and although variability exists in bronchodilator response between and within patients, patients with COPD do show a clinically meaningful response to bronchodilator therapy, administered alone or in combination.
Acknowledgments
The author thanks Anny Wu, PharmD, and Cynthia Gobbel, PhD, from Scientific Connexions (Newtown, PA) who provided medical writing support funded by AstraZeneca LP (Wilmington, DE).
References:
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Updated 2010. htttp://www.goldcopd.org. Accessed September 23, 2011.
2. Bleecker ER, Emmett A, Crater G, et al. Lung function and symptom improvement with fluticasone propionate/salmeterol and ipratropium bromide/albuterol in COPD: response by beta-agonist reversibility. Pulm Pharmacol Ther. 2008;21:682-688.
3. Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957-965.
4. Celli BR, Tashkin DP, Rennard SI, et al. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respir Med. 2011;105:1176-1188.
5. Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742-750.
6. Kesten S, Rebuck AS. Is the short-term response to inhaled beta-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest. 1994;105:1042-1045.
7. National Asthma Education and Prevention Program (NAEPP). Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 1997.
8. Gelb AF, Zamel N. Unsuspected psuedophysiologic emphysema in chronic persistent asthma. Am J Respir Crit Care Med. 2000;162:1778-1782.
9. Sitkauskienne B, Sakalauskas R, Malakauskas K, Lötvall J. Reversibility to a ß2-agonist in COPD: relationship to atopy and neutrophil activation. Respir Med. 2003;97:591-598.
10. Coultas DB. Passive smoking and risk of adult asthma and COPD: an update. Thorax. 1998;53:381-387.
11. Yawn B. Differential assessment and management of asthma vs chronic obstructive pulmonary disease. Medscape J Med. 2009;11:20.
12. Green RH, Brightling CE, Woltmann G, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum
neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875-879.
13. Wedzicha JA. Exacerbations: etiology and pathophysiologic mechanisms. Chest. 2002;121:136S-141S.
14. Soriano JB, Davis KJ, Coleman B, et al. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124:474-481.
15. Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest. 2004;126:117S-124S.
16. Snider GL. Distinguishing among asthma, chronic bronchitis, and emphysema. Chest. 1985;84:35S-39S.
17. Gold WM, Kaufman HS, Nadel JA. Elastic recoil of the lungs in chronic asthmatic patients before and after therapy. J Appl Physiol. 1967;23:433-438.
18. Ohar JA, Sadeghnejad A, Meyers DA, et al. Do symptoms predict COPD in smokers? Chest. 2010;137:1345-1353.
19. Rytila R, Helin T, Kinnula V. The use of microspirometry in detecting lowered FEV1 values in current or former cigarette smokers. Prim Care Respir J. 2008;17:232-237.
20. Stoloff SW. Diagnosis and treatment of patients with chronic obstructive pulmonary disease in the primary care setting: focus on the role of spirometry and bronchodilator reversibility. J Fam Pract. 2011;60(4 suppl):S9-S16.
21. Buffels J, Degryse J, Heyrman J, et al. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO study. Chest. 2004;125:1394-1399.
22. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948-968.
23. Report of the Committee on Emphysema American College of Chest Physicians. Criteria for the assessment of reversibility in airway obstruction. Chest. 1974;65:552-553.
24. Siafakas NM, Vermeire P, Pride NB, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1995;8:1398-1420.
25. American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202-1218.
26. O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1557-1565.
27. Ben Saad H, Préfaut C, Tabka Z, et al. The forgotten message from gold: FVC is a primary outcome measure of bronchodilator reversibility in COPD. Pul Pharmacol Ther. 2008;21:767-773.
28. Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121:1042-1050.
29. Tashkin D, Kesten S. Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest. 2003;123:1441-1449.
30. Calverley PMA, Burge PS, Spencer S, et al, for the ISOLDE study investigators. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659-664.
31. Donohue JF. Therapeutic responses in asthma and COPD. Chest. 2004;126:125S-137S.
32. Dorinsky PM, Reisner C, Ferguson GT, et al. The combination of ipratropium and albuterol optimizes pulmonary function reversibility testing in
patients with COPD. Chest. 1999;115:966-971.
33. Price DB, Yawn BP, Jones RCM. Improving the differential diagnosis of chronic obstructive pulmonary disease in primary care. Mayo Clin Proc. 2010;85:1122-1129.


