Community-Acquired MRSA: What You Need to Know
ABSTRACT: Staphylococcus aureus, specifically methicillin-resistant S aureus (MRSA), is now a ubiquitous pathogen that is a cause of significant morbidity and mortality. Community-acquired MRSA is an even bigger threat because of its increasing prevalence and propensity to spread in the general population. This article will review the epidemiology, pathogenesis, clinical manifestations, and management strategies pertaining to MRSA.
The word Staphylococcus has its origin in the Greek word that translates to a “bunch of grapes.” Alexander Ogston, a Scottish surgeon is credited with first describing the organism and elaborating on its role in sepsis and abscess formation in the late 1800s. German surgeon Friedrich Rosenbach later speciated the organism based on colony color. Staphylococcus was recognized as a major culprit in wound infections, especially during both World Wars, and has continued to be a major component in several infections, both in the community and healthcare settings.1
Microbiology, Epidemiology
Staphylococci are facultatively anaerobic gram-positive cocci, which are classically seen in clusters; they are nonmotile, do not form spores, and usually elaborate catalase. There are several species in the genus, of which the one that is germane to the scope of this article is Staphylococcus aureus.
In healthy individuals, S aureus is a common colonizer and resides in multiple sites on the integument and mucosa—the main site is the anterior nares, although additional sites (eg, the axilla and groin) are being recognized more frequently. Spread of the bacterium between individuals is thought to happen primarily via direct contact, which is particularly important in the context of the healthcare setting. The organism has also been shown to less commonly propagate through fomites and the airborne route.
Individuals who carry S aureus can be further divided into “persistent” and “intermittent” carriers. Certain underlying conditions—eg, hemodialysis, intravenous drug use, HIV, surgery, and diabetes—have been associated with higher rates of carriage. Nasal carriage and organism burden are known risk factors for future infections.2
According to the CDC, about one-third of the general population carries the Staphylococcus organism in their nares, usually with no evidence of disease; 2% are methicillin-resistant S aureus (MRSA) carriers.3 However, data is lacking on the numbers of skin and soft tissue infections (SSTI) caused by MRSA in the community.
A recent study published by the CDC showed that nearly 80% of the total numbers of invasive MRSA infections over the study period, in 2011, were in the community setting, which included both community-acquired infections and healthcare-associated outpatient infections; this number was significantly higher than in 2005.3 There was an overall significant decrease in the rate of invasive MRSA infections, however this decrease was most evident in the healthcare setting and much less so in the community setting. Furthermore, there were a higher proportion of people with intravenous drug use, HIV, and smoking histories in the cases with community-acquired MRSA (CA-MRSA) infections.3
MRSA PFGE strain type USA300 was first reported in the United States in 2000, soon becoming the dominant strain in CA-MRSA infections. It has caused outbreaks in multiple settings including daycare centers, prison populations, military personnel, and among men who have sex with men (MSM). It was initially thought to cause SSTI, but has also been reported in severe and invasive diseases, including bloodstream infections (BSI) and endovascular infections.
The USA300 has spread to South America, Europe, and Australia. These isolates carry the Panton-Valentine leukocidin (PVL) genes and the arginine catabolic mobile element. They have increasingly developed resistance to several antibiotics, including clindamycin, quinolones, tetracyclines, mupirocin, and macrolides. Furthermore, and perhaps of greatest concern, is the reduced susceptibility to vancomycin and daptomycin; resistance patterns for USA300 are now thought to be similar to that of the more drug resistant USA100 strain.4,5
Clinical Presentation
The various virulence factors possessed by this organism give S aureus the capability to form abscesses. In the community, S aureus commonly causes SSTI, including furuncles, abscesses, and purulent cellulitis. It can also cause pneumonia, especially in the cases of prior recent influenza infection, endovascular infections, and osteomyelitis. It can cause infection at remote sites by spreading through the bloodstream. The range of infections broadens to include mainly wound and postoperative infections, prosthetic joint infections, endovascular infections, catheter-associated BSIs, and healthcare-associated pneumonias in the healthcare setting.
S aureus bacteremia is a serious concern in the healthcare setting as it is a major cause of morbidity and mortality. It is a known causative agent in toxin-mediated diseases; these can be either directly or indirectly due to produced toxins. Staphylococcal scalded skin syndrome and staphylococcal toxic shock syndrome are both caused by toxins that are elaborated directly by the organism. Food poisoning caused by S aureus occurs due to ingestion of a pre-formed toxin and is therefore characterized by a very short incubation period of several hours prior to symptom onset.2,6
Antibiotic Resistance
During World War II, penicillin was the wonder drug that killed S aureus. However, some of the first resistant isolates were seen as early as 1944; MRSA first reared its head in the 1960s. Methicillin resistance occurs due to the formation of a different penicillin-binding protein called PBP2A, which is determined by the mecA gene. Healthcare-associated spread of MRSA has been a huge problem but is now on the decline. Nevertheless, CA-MRSA continues to be a major problem; as mentioned earlier, several outbreaks have been reported in prisons, daycare centers, among MSM, and military recruits, and these outbreaks occur in individuals with no underlying medical conditions.
Vancomycin has been the drug of choice for MRSA for several decades. However, strains that are only partially susceptible (vancomycin-intermediate S aureus) and resistant (vancomycin-resistant S aureus) have emerged over the past several years and are now a major threat, especially in the healthcare setting. Often, resistance to vancomycin is associated with resistance to other drugs like daptomycin.7
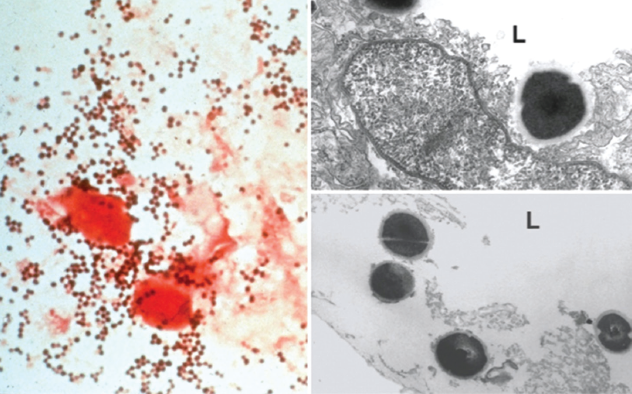
Figure. Gram staining and transmission electron microscopy of clinical samples of Staphyloccocus aureus.
Clinical Conditions and Their Management
This summary will focus on important infections that are seen commonly in the setting of CA-MRSA. The rate of serious complications in S aureus bacteremia is very high. This is especially true with endocarditis, which can occur in up to 40% of patients with S aureus bacteraemia.8
Skin and Soft Tissue Infections
SSTIs caused by CA-MRSA are a common entity encountered by the clinician. Since S. aureus is a common skin colonizer, it has a propensity to cause these infections by contiguous spread. As a general rule, the mainstay of treatment for a skin abscess is incision and drainage. For small or simple boils or abscesses, it suffices to only do the drainage procedure without antibiotic therapy.6 There are times when antibiotic therapy is appropriate. These conditions include severe disease, rapid deterioration, systemic signs/symptoms, multiple abscesses, underlying immune compromise, lack of clinical improvement with incision and drainage, or association with septic phlebitis.6,7
- Purulent cellulitis. S aureus also causes purulent cellulitis. In the outpatient setting, when treating patients for purulent cellulitis, it is recommended to provide empiric antibiotic therapy for MRSA while awaiting culture results. The role of CA-MRSA in nonpurulent cellulitis is unclear, and this condition would warrant empiric therapy for β-hemolytic streptococci. In this case, using trimethoprim/sulfamethoxazole (TMP/SMX) may alone would not achieve adequate coverage for β-hemolytic streptococci, which is the more likely pathogen. Cultures are essential in those who have purulent drainage, in those with no response to initial antibiotic therapy, or if the clinician is concerned that there might be an outbreak in the community.9
- Streptoccocus impetigo. Impetigo is a superficial infection that involves only the stratum corneum of the epidermis. It is more common in the pediatric population. S aureus is a major etiologic agent in SSTIs, including impetigo, more so than β-hemolytic streptococci.10 The lesion is an erythematous macular area that then changes into vesicles filled with cloudy fluid. It is usually associated with concomitant local lymphadenopathy, is highly contagious, and resolves with topical therapy in most cases.
Lesions
S aureus can cause several different types of SSTIs as mentioned above. These infections are characterized by the anatomic component involved, and are most commonly diagnosed clinically. The fundamental underlying lesion is an abscess or pus formation, and the infection can range from being superficial to involving the deeper tissues down to the muscles and fascia.
- Folliculitis. This is a lesion involving the hair follicle and its surrounding area. It presents as multiple, raised erythematous lesions, which are painful, and contain a hair follicle at the center. Local antiseptics usually lead to resolution of the lesions.
- Furuncles and carbuncles. Boils/furuncles present in the axillae, groin, and perirectal areas because they are basically an extension of the infection in the hair follicles. They usually occur on the face, axillae, neck, and buttocks. The lesion is a painful, erythematous nodule that then indurates and can discharge a small amount of pus if it drains. Local treatment is usually sufficient. CA-MRSA is notorious as an etiologic agent for furuncles.
The lesions—thought to occur in CA-MRSA that are positive for PVL—can occur in outbreaks, and can evolve into abscesses or purulent cellulitis. This gene encodes a potent toxin, which is putatively held responsible for the increased virulence of the strains that carry it.4 Although rare, when the lesions are present around the upper lip or nares, these lesions can rapidly deteriorate and cause septic thrombophlebitis of the intracerebral veins; therefore, when present in this location, the lesions should be treated with intravenous antibiotics to prevent this dreaded complication.
Carbuncles refer to a deep infection that involves multiple hair follicles and then spreads to involve subcutaneous tissues. The usual location is the base of the neck. Systemic signs and symptoms may occur, and the infection can also spread into the bloodstream.
- Hydradenitis suppurativa. Hydradenitis is a chronic, inflammatory skin disease with pus formation in the apocrine sweat glands in the axilla, perineum, and groin. It presents as groups of furuncles that usually drain spontaneously but may leave scarring in the area. This is a difficult condition to treat because of multiple recurrences in some patients. Usually the lesions respond to topical therapy, eg, topical clindamycin, which may be useful in part because of its anti-inflammatory properties.
- Mastitis. Breastfeeding mothers are at risk for developing mastitis, usually 2 to 3 weeks after delivery. The condition is diagnosed clinically and presents with tenderness located in a focal area in 1 breast, usually with systemic signs of fever; it can sometimes lead to abscess formation. Mastitis is managed with analgesics and changing the technique of breastfeeding in order to alleviate milk stasis.
The recommendation is to continue breastfeeding; antibiotics are necessitated when there are systemic signs and symptoms. Breast abscesses are treated with incision and drainage.
- Surgical site infection. Clinically, the infections present with erythema, pain, and swelling around the surgical site incision that starts at least 48 hours post-surgery. A high index of suspicion and good examination are essential in diagnosing these infections. Depending on the severity of the infection, treatment may include either oral or parenteral antibiotic therapy, and debridement of the wound or removal of prosthetic materials may be necessary. Underlying comorbidities such as diabetes or vascular insufficiency contribute as risk factors by delaying healing.
Surgical site infections have been shown to occur in up to 2.6% of surgeries in the United States.3 Community-acquired S aureus is a frequent culprit, as are coagulase-negative staphylococci, except after abdominal surgery when gram-negatives and enterococci are more common. It has been shown that nasal colonization with S aureus is a strong risk factor for infection in these patients. Studies have shown that 3 strategies are effective at eliminating nasal carriage: topical antibiotics, bacterial interference, and systemic antibiotics. Mupirocin has been shown to be 97% effective at reducing nasal carriage of S aureus.11-13
- Fasciitis. Fasciitis can occur due to spread from a BSI; it can present with severe pain out of proportion to physical exam findings. Necrotizing fasciitis is a severe condition that warrants immediate attention and surgical intervention along with parenteral antibiotic therapy. The severity of illness may be masked because the infection may present with minimal local signs. CA-MRSA, as well as Streptococcus pyogenes, have been implicated as causative agents.
Management. As mentioned earlier, small or localized lesions can usually be managed with topical therapy and local disinfection. Mupirocin should not be used for treatment, and should be reserved for decolonization.13 The use of clinical judgment and individualizing therapy to the patient is, as always, important. The clinician must also decide who needs to be hospitalized for treatment of an SSTI based on underlying comorbidities and severity of the illness.
Empiric antibiotic therapy in the healthcare setting must take into account that the organism might be resistant to the usual antibiotics. However, in the CA-MRSA setting, clindamycin or TMP/SMX is usually adequate, even though the rates of resistance are increasing. There have always been concerns of possible inadequate treatment of S pyogenes, which commonly causes SSTIs with TMP/SMX, although these concerns are now being challenged.14 Doxycycline or minocycline have activity against MRSA and may be useful in patients with allergy to sulfa drugs.
Recurrent SSTIs
Special mention needs to be made of recurrent SSTIs caused by CA-MRSA. All patients who are treated for an SSTI should be given education on wound care and personal hygiene—including regular hand washing, cleaning and bathing, avoiding sharing of personal care paraphernalia, and domestic cleanliness—to prevent recurrence.
In cases where the patient has recurrences in spite of optimal hygienic measures or in those households where close contacts become repeatedly infected, decolonization is another strategy that can be used. Although multiple regimens have been evaluated, most patients become recolonized after several months.
Note: A common regimen includes nasal decolonization with mupirocin twice a day for a duration of 5 to 10 days along with topical total-body decolonization with an antiseptic, such as chlorhexidine washes.
Systemic antibiotics are not recommended for decolonization.
Patients in whom an MRSA infection has not been documented by cultures need screening cultures prior to decolonization. However, if the patient has already had a prior MRSA infection, there is no need for doing nasal cultures.9
Bloodstream Infections
BSI refers to one or more positive blood cultures, in association with concomitant systemic signs/symptoms. The rates of BSIs have been on the rise, with gram-positive organisms becoming the most common culprits. Rates of S aureus are affected by the increased use of intravascular devices (eg, central lines, pacemakers, etc), injection drug use, comorbidities such as diabetes, and the use of immunosuppressive medications.
There are 2 categories of BSI: community-acquired, which happens in the community or within 48 hours of hospitalization, and nosocomial BSI, which happens 2 or more days after admission. However, this delineation is now blurry because many patients receive parenteral therapy in the outpatient setting. Some authors therefore suggest that the name should be changed from community-acquired to community-onset bacteremia.
Community-onset bacteremia can then be further divided into community-associated and healthcare-associated. Healthcare-associated community-onset BSI is similar to nosocomial BSI in that it has risk factors, such as parenteral devices, hemodialysis, and surgical history.
Community-associated BSI happens in patients with no underlying comorbidities and is usually caused by more susceptible strains of the organism. There is usually a nidus for infection that can be determined, which can include SSTI, bone or joint infections, endovascular infections such as endocarditis, or deep tissue abscesses.6
Management. S aureus is an organism that is to be taken very seriously, even with just a single positive blood culture. In this case, prompt follow-up cultures, antibiotic therapy, and finding the source of infection are absolutely essential. As many as 30% of patients with S aureus BSI go on to have complications from seeding of the infection to remote sites, especially if they have indwelling prosthetic material. If the cultures remain positive for over 96 hours after appropriate therapy, the index of suspicion for these complications should increase.
The main aim should be to eliminate the nidus of infection. If this is not done, the likelihood of the infection recurring increases. This becomes difficult in cases where there is a nonremovable, implanted prosthetic device, such as an indwelling long-term intravascular catheter. If the line is clearly the source, the bacteremia clears promptly, and there are no risk factors for complications, 2 weeks of therapy may suffice. Deep-seated infections require longer duration of therapy, with adjunctive therapy in some cases.
Infective Endocarditis
One of the most dreaded consequences of S. aureus bacteremia, infective endocarditits is characterized by destruction of the valve tissue, abscess formation, rapid deterioration if left undetected, and spread of the organism to remote sites including (but not limited to) the CNS, kidneys, spleen, joints, and muscles. Specific examination findings, such as a new murmur (especially diastolic), are a very important clue that should not be missed by the clinician.
S aureus can cause large vegetation that have a propensity to break off into the circulation and seed remote sites. Septic emboli to the periphery (Janeway lesions) and immune complex mediated phenomena in the periphery, such as Osler’s nodes, can also be present but these are subtler. Nearly 30% of patients have CNS involvement (eg, cerebritis, mycotic aneurysms, emboli). New-onset cardiac failure is a red flag and is an indication for surgical valvular replacement. Prompt recognition and treatment is critical and failure to do so may result in death.6
Pulmonary Infections
Pneumonia due to S aureus usually occurs after viral infections, such as influenza, and is more common in those who are elderly or have underlying comorbidities.
Osteoarticular Infections
CA-MRSA is emerging as a causative agent in osteomyelitis, which it can cause either by hematogenous seeding or from contiguous spread. It is also implicated in early infections after prosthetic joint replacements, and this is more common in patients who have a revision. S aureus is also an important cause of septic arthritis both in adults and children. Diabetes and rheumatoid arthritis are known risk factors. It is also implicated in pyomyositis and septic bursitis.
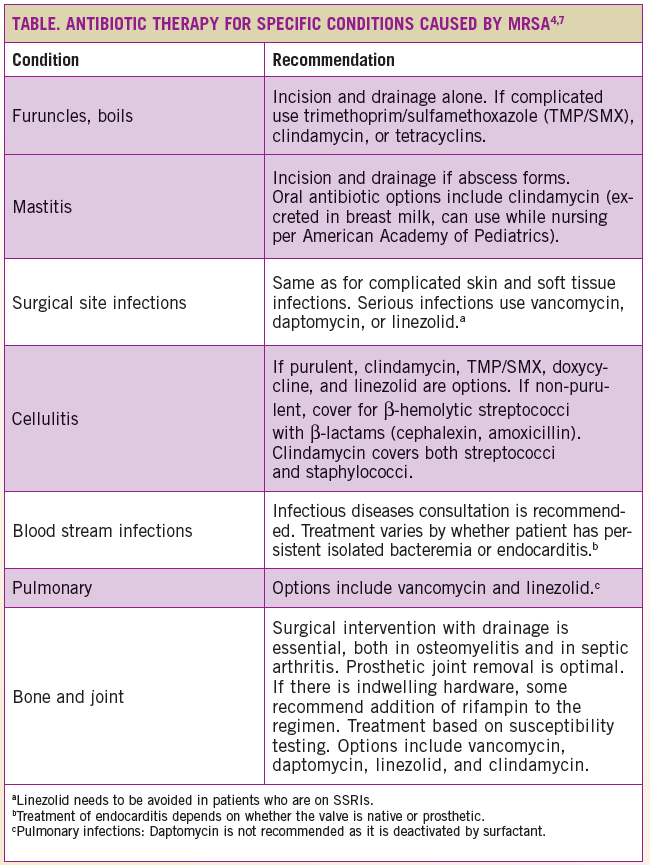
Recommendations for antibiotic therapy are outlined in the Table.
CA-MRSA is a pathogen that is of utmost importance for primary care providers to familiarize themselves with because of its increasing incidence and its associated morbidity and mortality. Proper diagnosis and involvement with the infectious disease consultant when necessary is an important step in managing these infections appropriately.
Donald P. Levine, MD, is a professor of medicine at Wayne State University in Detroit, MI. He has had a long interest in the management of staphylococcal infections and is a member of the consensus panel of the Infectious Disease Society of America for the management of MRSA infections.
Ambika Eranki, MD, is a senior infectious disease fellow in the division of infectious disease, department of medicine at Wayne State University in Detroit, MI.
References:
- Ekkelenkamp MB, Rooijakkers SHM, Bonten MJM. Staphylococci and micrococci. In: Infectious Diseases. 3rd ed. Philadelphia, PA: Mosby Elsevier; 2010:165,1632-1644.
- Dantes R, Mu Yi, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970-1978.
- Centers for Disease Control and Prevention. Methicillin resistant Staphylococcus aureus (MRSA) infections. www.cdc.gov/mrsa/tracking/index.html. Accessed January 1, 2014.
- Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest.. 2007;87(1):3-9.
- Tenover FC, Goering RV. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother. 2009;64(3):441-446.
- Que YA, Moreillon P. Staphylococcus aureus (including staphylococcal toxic shock). In: Mandell G. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2009:195,2543-2578.
- van Hal SJ, Paterson DL, Gosbell IB. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naïve patient—a review of the literature. Eur J Clin Microbiol Infect Dis. 2011;30(5):603-610.
- Fowler WG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteraemia. Arch Intern Med. 2003;163(17):2066-2072.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):18-55.
- Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a US population: a retrospective population-based study. BMC Infect Dis. 2013;13:252.
- Kalra L, Camacho F, Whitener CJ, et al. Risk of methicillin-resistant Staphylococcus aureus surgical site infection in patients with nasal MRSA colonization. Am J Infect Control. 2013;41(12):1253-1257.
- Perl TM. Prevention of Staphylococcus aureus infection among surgical patients: beyond traditional perioperative prophylaxis. Surgery. 2003;134(5 suppl):10-17.
- Hetem DJ, Bonten MJ. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect. 2013;85(4):249-256.
- Bowen AC, Lilliebridge RA, Tong SYC, et al. Is Streptococcus pyogenes resistant or susceptible to trimethoprim-sulfamethoxazole? J Clin Microbiol. 2012;12:4067-4072.


