Peer Reviewed
Evaluation and Management of Hearing Loss in Older Adults
Introduction
Hearing impairment is an extremely prevalent problem among older adults that can impact all domains of life, including cognitive, social, physical, and emotional. Identification and treatment can improve overall quality of life as well as functional capacity. Primary care providers play an essential role in identifying hearing loss and ensuring that patients are treated appropriately. This article discusses the epidemiology, risk factors, consequences, and etiology of hearing loss in the older patient. Information on screening techniques, hearing evaluation, and treatment options including hearing aids and cochlear implantation is also included.
Epidemiology and Risk Factors
The World Health Organization estimated that 278 million people had a mild-to-moderate hearing impairment in 2005.1 In the United States, there are 28-32 million Americans of all ages with some degree of hearing impairment.2,3 Other reports estimate that 25% of people 51-65 years of age, 10-33% of people 66-75 years of age, 25-60% of people 76-85 years of age, and 50-80% of people over 85 years of age have hearing impairment.4 This makes hearing loss the third most common chronic disease of the elderly, following hypertension and arthritis.2,5 As these numbers demonstrate, hearing loss increases with age. It is also associated with male gender, non-Hispanic white race, lower level of education, lower socioeconomic class, prior service in the military, occupational noise exposure, heavy alcohol use, tobacco use, diabetes mellitus, and cardiovascular disease.6-8
Consequences of Hearing Impairment
 The high prevalence of hearing impairment is of clinical importance because of its association with social, emotional, cognitive, and physical impairments (Table I). Adults with hearing impairment are more likely to have depression, anxiety, paranoia, psychosis, insecurity, social isolation, and family discord. In terms of physical limitations, adults with hearing impairment are more likely to have difficulties with activities of daily living and with instrumental activities of daily living.9,10 Hearing loss also impacts the physician-patient relationship. Physicians may find that taking a medical history is a challenge when a patient does not clearly understand questions. In addition, patient education can be impaired by misunderstandings, which can be dangerous when they limit a patient’s understanding of a diagnosis and/or prescribed treatments. Finally, hearing impairment is associated with an increased risk of dementia, and the severity of dementia has been misclassified in patients with undetected and untreated hearing loss.2
The high prevalence of hearing impairment is of clinical importance because of its association with social, emotional, cognitive, and physical impairments (Table I). Adults with hearing impairment are more likely to have depression, anxiety, paranoia, psychosis, insecurity, social isolation, and family discord. In terms of physical limitations, adults with hearing impairment are more likely to have difficulties with activities of daily living and with instrumental activities of daily living.9,10 Hearing loss also impacts the physician-patient relationship. Physicians may find that taking a medical history is a challenge when a patient does not clearly understand questions. In addition, patient education can be impaired by misunderstandings, which can be dangerous when they limit a patient’s understanding of a diagnosis and/or prescribed treatments. Finally, hearing impairment is associated with an increased risk of dementia, and the severity of dementia has been misclassified in patients with undetected and untreated hearing loss.2
Treatment of the hearing loss appears to improve these limitations. Studies demonstrate improved self-esteem, greater independence, healthier relationships, and improved mental health with the use of hearing aids. Yueh et al11 demonstrated improvement in Hearing Handicap Inventory for the Elderly-Screening Version (HHIE-S; described below) scores with the use of hearing aids. Quality-of-life measures can also be improved with auditory rehabilitation.12
Despite these benefits, many adults with hearing impairment are not identified or are not treated adequately. One review estimated that only 9% of internists screen their patients who are 65 years of age or older for hearing loss.13 This lack of screening leads to a lack of diagnosis and treatment. Only 20-25% of adults with hearing loss amenable to amplification receive a hearing aid.13,14 Additionally, 25-40% of patients with hearing aids underuse their device and 29% no longer use their device.15,16 Common reasons for the underuse or discontinuation of hearing aid use include unreasonable expectations, stigma associated with hearing aids, difficulty using the device, and associated costs. On the other hand, severity of hearing loss, self-perceived handicaps, age, and poor word recognition scores are associated with increased hearing aid use. Primary care providers can help to improve these rates by identifying individuals who would benefit from hearing aids, setting reasonable expectations for patients with hearing aids, and continuing to inquire about hearing aid use among those patients.
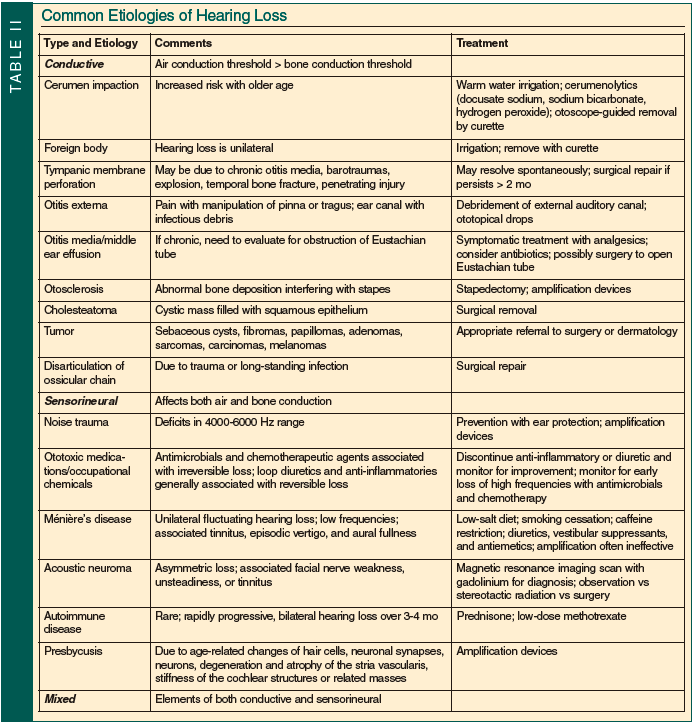
Etiology of Hearing Loss
Hearing loss is generally divided into three types: conductive; sensorineural; and mixed (Table II). Understanding the anatomy and physiology of the ear is essential when considering common causes of hearing loss. The pinna and external auditory canal form the outer ear. Together they collect sound from the environment and increase the sound level by 12-15 decibels. The middle ear, comprised of the tympanic membrane, ossicles (malleus, incus, and stapes), middle ear cavity, and Eustachian tube, serves as a transformer that changes sound waves into physical vibrations. This process is most efficient at frequencies of 500-4000 hertz and the energy is amplified by 25-30 decibels. The oval window is the connection between the middle ear and the inner ear. The oval window can be stimulated by energy traveling through the outer and middle ear or through the bones of the skull, and sets the cochlear fluid in motion. This pressure wave in the cochlea induces a wave of the basilar membrane (1 of 2 walls that separate the vestibular canal, tympanic canal, and cochlear duct). The basilar membrane has different characteristics at the base (narrow and stiff) and apex (wide and flexible) that cause different frequencies of waves to peak at different locations. The location of the wave’s peak determines which nerve fibers are stimulated and, as a result, what pitch is perceived. High-frequency tones peak at the base of the cochlea and lower frequencies peak at the apex.
Conductive Hearing Loss
Conductive hearing loss results when the transmission of sound from the air to the inner ear is prevented. Because the inner ear remains intact, hearing via bone conduction remains intact. Thus, conductive hearing loss is diagnosed by an air-bone gap where the threshold for air conduction is greater than that for bone conduction. The size of the air-bone gap indicates the severity of impairment: 25 decibels is “mild”; 40 decibels is “moderate”; and 60 decibels is “severe.” Causes of conductive hearing loss include cerumen impaction, obstruction from a foreign body, tympanic membrane perforation, otitis externa, otitis media, middle ear effusion (with possible blockage of the Eustachian tube), otosclerosis, cholesteatoma, tumor, Paget’s disease of the bone, and disarticulation of the ossicular chain (due to trauma or long-standing infection).
With advancing age, cerumen becomes drier and more tenacious, increasing the risk for cerumen impaction (and subsequent interference with hearing aids); impaction is found in up to 30% of elderly patients with hearing loss.13 Smeeth et al6 found that after wax removal, nearly half of adults with wax impaction passed the whispered voice test (described below), highlighting the importance of evaluation and treatment of cerumen impaction. Tympanic membrane perforations can be due to chronic otitis media or trauma including barotrauma, explosions, penetrating injury, or temporal bone fracture. Otosclerosis is abnormal bone deposition near the base of the stapes; it is found histologically in 10% of Caucasians, but hearing loss develops in only 1% of these individuals.13 Otosclerosis classically presents as a progressive bilateral conductive loss in a middle-aged Caucasian woman with a family history of hearing loss. A cholesteatoma is a cystic mass that develops in the middle ear or mastoid cavity that is filled with trapped squamous cell epithelium. As it slowly grows, a cholesteatoma can destroy the surrounding structures. Erosion into the ossicles occurs frequently in the setting of cholesteatomas and often presents with chronic drainage and conductive hearing loss.13 Paget’s disease of the bone can affect the inner ear, leading to a conductive loss. Tumors causing conductive loss are rare and include sebaceous cysts, fibromas, papillomas, adenomas, sarcomas, carcinomas, and melanomas.
Sensorineural Hearing Loss
Sensorineural hearing loss results when the cochlea is unable to transform air or bone vibrations into neural activity. Both bone conduction and air conduction are reduced. Causes of sensorineural hearing loss include noise trauma, ototoxic medications or occupational chemicals, genetics, vascular disease, diabetes mellitus, cigarette smoking, autoimmune disease, auditory nerve tumors (eg, acoustic neuroma), neuronal loss, Ménière’s disease, idiopathic, and presbycusis.
Noise exposure causes both mechanical and metabolic damage to the cochlea; associated hearing loss typically presents with deficits in the region of 4000-6000 hertz with a “notch” pattern on an audiogram. Individuals with a history of noise exposure are also more likely to develop hearing loss at other frequencies as they age. Because noise can be so harmful, United States federal law requires baseline and annual audiometry and provision of hearing protectors for the estimated 5 million workers who are exposed to hazardous levels of noise (defined as 8-hr time-weighted average sound level of 85 dB).17 Simple ear plugs can reduce sound level by 15-25 decibels.
Ototoxic medications include antimicrobials (aminoglycosides, erythromycin, and vancomycin), chemotherapeutic agents (cisplatin, carboplatin, and vincristine sulfate), loop diuretics (furosemide, ethacrynic acid), and anti-inflammatories (aspirin, quinine). Aminoglycosides and chemotherapy are well documented to cause irreversible damage. Initially, high frequencies are affected, and therefore if impairment is detected early, progression to loss in the conversational range (range of normal speaking) can be prevented with cessation of the medication.8,13 Damage from anti-inflammatories and diuretics is generally reversible with discontinuation of the medication.8
A higher prevalence of hearing loss was seen in adults with diabetes (age, 25-69 yr) in the National Health And Nutrition Examination Survey (NHANES) I (1971-1973) and NHANES 1999-2004. Diabetic hearing loss affects low frequencies and is thought to be due to a process similar to diabetic retinopathy, nephropathy, and neuropathy.5
The role of tobacco and alcohol use in hearing loss remains unclear. However, smoking does appear to increase the risk of noise damage at high frequencies and generally is thought to increase the risk of hearing loss.18
Autoimmune hearing impairment is rare; it usually presents as a rapidly progressive loss over a few months and can be associated with vertigo and disequilibrium. The diagnosis is confirmed with response to prednisone or other immunosuppressive medications. Auditory nerve tumors are a rare cause of hearing loss that present as either a unilateral loss or an asymmetric loss. Associated symptoms include facial nerve weakness, unsteadiness, and tinnitus. A magnetic resonance imaging scan with gadolinium is the gold standard for diagnosis. Ménière’s disease presents as a unilateral, fluctuating, low-frequency hearing loss and is often associated with aural fullness, tinnitus, and episodic vertigo.
Presbycusis is the most common cause of sensorineural hearing loss in older adults that results from age-associated changes in the inner ear.10 Presbycusis presents as a gradual, bilateral, sensorineural hearing loss that is more prominent in the high frequencies. Four types of presbycusis have been described, but most likely presbycusis is caused by a combination of these types. First, sensory presbycusis is associated with hair cell loss. Second, neural presbycusis is due to decreased auditory nerve output. Decreased output is due to asynchronous and poorly synchronous neural activity caused by degeneration of hair cells, neuronal synapses, and neurons. Hair cell loss also leads to secondary degeneration of the central pathways, which is more common than primary degeneration. Third, strial or metabolic presbycusis is caused by degeneration and atrophy (up to 30%) of the stria vascularis. This is caused by microvascular changes such as thickening of the capillaries in the stria vascularis. These changes modify the electrical composition of the endolymph, which is required to produce and transmit action potentials. Fourth, cochlear conductive presbycusis is not clearly understood but is related to stiffness of the cochlear structures or related masses.8
Screening
Rates of routine screening for hearing impairment in older adults are quite low, despite recommendations from the United States Preventive Services Task Force, the Canadian Task Force on Preventive Health Care, the American Academy of Family Physicians, and the American Speech-Language-Hearing Association. Cohen et al19 found that approximately 40% of primary care physicians did not routinely screen for hearing impairment. Newman and Sandridge2 reported that only 16% of adults 65 years of age or older were screened at their last physical examination.
A number of tests have been used for screening, such as the global question test, whispered voice test, HHIE-S, and audioscope (Table III). A single global question such as, “Do you have a hearing problem now?” was found to have a sensitivity of 71% and a specificity of 72% when both “yes” and an equivocal response were interpreted as positive.4 It is a moderately accurate test with a likelihood ratio of 2.2 for a positive test and 0.45 for a negative test.
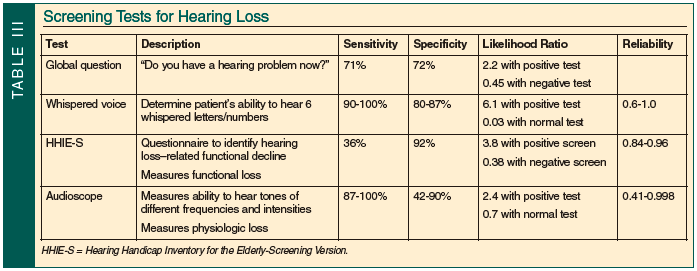
The HHIE-S is a 10-item questionnaire that evaluates for a functional decline in hearing or a hearing handicap.20,21 In one study, patients with a moderate-to-severe hearing loss were 34 times more likely to have a positive HHIE-S screen, and patients with a mild hearing loss were six times more likely to have positive HHIE-S screen.9 In another study, the HHIE-S had a sensitivity of 36% and a specificity of 92%.22 The HHIE-S has excellent test-retest reliability, with Pearson correlation of 0.84 when given in written form and 0.96 when given orally. Accuracy is fair, with a likelihood ratio of 3.8 for a positive screen; a negative screen is less useful, with a likelihood ratio of 0.38.4
The whispered voice test measures the ability to identify a whispered series of letters and numbers. This simple maneuver has a sensitivity of 90-100% and specificity of 80-87%.23 Reliability and reproducibility vary considerably, with correlations ranging from 0.6 to 1.0 with different types of examiners. The variation is due to difficulty standardizing the test since it is impossible to standardize the loudness of the whisper. In addition, selection of the letters and numbers presented change the difficulty of the test due to the different frequencies of different letters (ie, consonants are a higher frequency).23 Despite the lack of standardization, it is an accurate test, with a likelihood ratio of 6.1 that the patient has a greater than 30-decibel impairment if positive and a likelihood ratio of 0.03 if normal.4
The audioscope measures physiologic hearing loss by delivering tones at 500, 1000, 2000, and 4000 hertz, and uses intensities of 20, 25, and 40 decibels to determine which tones are heard by the patient. Interobserver reliability is variable between studies, with correlations ranging from 0.41 to 0.998. The audioscope has a sensitivity of 87-100% and a specificity of 42-90%.4 A different study reports a sensitivity of 94% and specificity of 69-80%.13 With a normal test, the likelihood of hearing impairment is decreased by 0.7 and with a positive test increased by 2.4.
Hearing Evaluation
Evaluation of hearing loss in the primary care setting begins with a thorough history and physical examination. The history should characterize the onset of the hearing loss and its change over time; identify associated symptoms such as tinnitus, aural fullness, and vertigo; ascertain risk factors for hearing loss, such as noise exposure, diabetes, smoking, family history, autoimmune disease, and cardiovascular disease; survey for ototoxic medication use; and ask about ear infections and trauma. The physical examination begins with an assessment of the outer ear, looking for signs of infection, obstruction of the external auditory canal, and bleeding or drainage. Inspection of the tympanic membrane should include pneumoscopy; opacification of the tympanic membrane is often seen in normal aging. Further evaluation of the head and neck, including cranial nerve testing, should also be performed.
Special tests include the Weber and Rinne tuning fork tests, which should be performed using a 512-hertz tuning fork.24 The Weber test is done by holding a vibrating tuning fork on the apex of the patient’s forehead and asking the patient where the sound is heard. If there is no hearing loss, the sound will not lateralize. If there is a conductive loss, the sound will localize to the ear with the loss. If there is a sensorineural loss, the sound will localize to the ear opposite the loss. Of note, the test will be normal if there is bilateral loss because the test is based on a difference between the ears. The Rinne test compares air conduction and bone conduction. Normally, air conduction is greater than bone conduction. The test can be done by loudness comparison or threshold technique. To compare loudness, the patient is asked when, of the following scenarios, the tuning fork is heard louder: when held against the mastoid or when held near the ear canal. For the threshold technique, the tuning fork is held against the mastoid bone until it can no longer be heard. It is then moved near the ear canal, and the patient is asked if the sound is again heard. The opposite ear should be masked by rubbing the tragus during the test. In a normal test, the sound is heard louder or longer when held in front of the ear canal. If the opposite is true, there is a conductive hearing loss on that side. The Weber and Rinne tests have poor reliability and accuracy, making them inappropriate for screening tests, but they remain helpful in evaluating a patient with a known hearing loss.
A referral to an audiologist or otolaryngologist should be made for sudden hearing loss, unilateral or significantly asymmetric hearing loss, hearing loss following trauma to the ear, perforated tympanic membrane, persistent ear drainage, when there is no obvious treatable etiology, or when screening is positive. A referral to a specialist can help clarify the etiology of the hearing loss and/or assess the need for surgical intervention.
A full audiometric examination includes audiogram with pure-tone thresholds, word recognition tests, speech reception thresholds, acoustic reflexes, and tympanometry. A pure-tone threshold is the intensity where a patient is able to hear the tone 50% of the time and is often averaged over different frequencies. Thresholds are done for both bone and air conduction. Average pure-tone thresholds define hearing loss severity as follows: normal (0-25 dB); mild (25-40 dB); moderate (40-55 dB); moderately severe (55-70 dB); severe (70-90 dB); and profound (> 90 dB). As a reference for these tests, conversational speech typically occurs at frequencies (or pitches) of 500-3000 hertz and at intensities (or loudness) of 45-60 decibels.
Treatment
Treatment of hearing loss begins with prevention. Avoiding ototoxic exposures is the best method to prevent hearing loss. In addition, avoidance of loud noises and use of ear protective equipment are essential to prevent noise-induced hearing loss. Ototoxic medications should be used cautiously and screening for high-frequency loss should be considered to allow for the discontinuation before the frequencies of human hearing are affected. Smoking cessation should also be encouraged. Antioxidant therapy may help prevent ototoxic medication–induced hearing loss.25
At times, treatment of conductive hearing loss is straightforward (ie, removal of cerumen impaction, treatment of infection, repair of perforated tympanic membrane). Certain etiologies of sensorineural hearing loss have specific therapies, but many causes such as presbycusis are irreversible and require amplification therapy, which will be reviewed here.
The first step of treatment is audiometric rehabilitation, either formal or informal, to counsel patients and the patients’ families on strategies to communicate more easily. Simple strategies include catching the person’s attention before talking, eliminating (or minimizing) background noise, ensuring good light and facing the speaker to maximize lipreading, using gestures, and repeating or rephrasing what was said to prevent misunderstandings. A recent randomized, controlled trial demonstrated a decrease in communication and activity limitations with a rehabilitation program.12 However, both the control group and the treatment group showed improvements in quality of life, underscoring the importance of socialization to quality of life.
There are many options for amplification devices available (Table IV). Personal amplifiers are pocket-sized and have a microphone connected to headphones. Many devices are available to assist with specific activities, such as telephone amplifiers, visual alert devices for alarms (eg, smoke alarms, alarm clocks), and infrared systems for home television.

Hearing aids are the most common treatment for hearing impairment.26 Most patients with a documented hearing loss (ie, usually a pure-tone threshold of ≥ 40 dB) and difficulty with communication will be candidates for a hearing aid. Hearing aids improve social, cognitive, emotional, and communication function. They have also been found to be cost-effective, with a cost per health quality–adjusted life year of $9702 in men and $13,615 in women.16
The decision to use a hearing aid is generally made in consultation with an audiologist who discusses expectations and what type of hearing aid would be suitable for the individual patient. Important considerations include the nature and degree of hearing loss, the patient’s motivation, the patient’s ability to adapt to the use of the device, the patient’s dexterity and ability to manipulate the device, and the patient’s finances. There is an adjustment period and learning curve to using a hearing aid that requires regular use to allow for central adaptation. The audiologist fits the hearing aid and makes adjustments to suit the patient’s needs and to correct problems. Two examples of the tools used by audiologists in this process include the Hearing Aid Selection Profile and the Client-Oriented Scale of Improvement. This process of initial fitting and adjustments is time consuming and requires patient compliance and motivation. In most states, patients are able to return hearing aids within 30 days if they are not satisfied.
Hearing aids come in many different types and styles (Table IV). Behind-the-ear aids have a piece behind the ear that connects by a tube to a mold within the ear. This type of device allows for a wide range of features and easy manipulation, and is appropriate for mild-to-profound hearing loss. In-the-ear aids fill the entire concha and project into the canal. Such devices have a number of possible features and are fairly easy to manipulate. In-the-ear aids are appropriate for mild-to-severe hearing loss. In-the-canal aids are smaller and sit in the canal with only slight protrusion into the concha. These devices have fewer features and a smaller battery, and require more dexterity for manipulation. In-the-canal aids are appropriate for mild-to-moderate hearing loss. Completely-in-the-canal aids are the smallest and most cosmetic, but also have the fewest features and the smallest battery, and require the most dexterity for manipulation. These devices are appropriate for mild-to-moderate hearing loss.
The quality of analog (standard) and digital (programmable) hearing aids is similar, but digital aids have programs (eg, background noise suppression, feedback management, telecoil for phone use) that can provide improved satisfaction. A study of Veterans with symmetric bilateral, mild-to-moderate sensorineural hearing loss showed improvement in HHIE-S scores by 17 points with standard hearing aids and by more than 31 points with programmable aids (P = 0.01).11 In this study, patients were willing to pay $800 (ie, 29% of monthly income) for a standard aid and $2240 (ie, 78% of monthly income) for a programmable aid.11 This underscores the potential benefits of hearing aid programming. Unfortunately, cost is often a barrier to hearing aid use, as Medicare and most private insurance companies do not pay for the device (although they may cover the audiometric evaluations).4 Overall, there is not a specific type of hearing aid that is best for everyone; the choice depends on each individual patient’s needs.
In those with profound hearing loss, hearing aids may not provide benefit. In these patients, cochlear implants may be a consideration. Although implants are most successful when used in young children, their use is growing among older adults. Cochlear implants are generally used in the setting of moderate-to-profound, bilateral, sensorineural hearing loss. A cochlear implant has both internal and external devices. An electrode array is implanted into the cochlea and a receiver-stimulator is implanted under the skin. A headset is worn behind the ear that transmits signals to the implanted receiver. The receiver then digitizes the sound and transmits it to the electrode array where the auditory nerve fibers are directly stimulated. Unlike hearing aids, this system bypasses the sensory hair cells.
Cochlear implantation has been associated with improved mood, decreased loneliness and depression, better self-esteem, increased independence, higher speech recognition scores, and improved communication.22,26 Migirov et al22 found that 13 out of 20 patients were able to use the telephone and that 4 out of 20 patients were able to enjoy music following cochlear implantation when they were unable to do so prior to the procedure. Complications of the surgery are rare and are not increased in elderly patients as compared with younger patients. Complications include foreign body reaction, facial nerve paralysis, vertigo/dizziness, seroma, wound infection, device failure, and cerebrospinal fluid leak.
Medicare and many private insurance companies pay for the procedure. Older adults who meet the criteria for cochlear implants, including those with a willingness to participate in intensive postoperative follow-up and who have no contraindications to general anesthesia, should be referred to otolaryngology for further evaluation.26 An intensive training program is necessary to “teach” the patient how to hear with an implant. Such training is critical to optimal functioning. A patient’s ability to participate fully in these activities must be established prior to considering cochlear implantation. In general, the older the patient, the less the anticipated benefit from cochlear implantation. Older adults with cognitive impairment are unlikely to be able to perform the necessary training.
Summary
Hearing loss is very common in the elderly, and leads to impairments in many domains of life. Identification through screening and a thorough evaluation by primary care physicians is simple and important. Treatment with amplification devices and cochlear implants can improve hearing, quality of life, and overall functional ability in older adults.
The authors report no relevant financial relationships.
Dr. Weener is from the Department of Internal Medicine, University of Michigan Medical School, Ann Arbor; Dr. Zacharek is from the Department of Otolaryngology, University of Michigan Medical School, Ann Arbor ; and Dr. Malani is from the Department of Internal Medicine, Divisions of Geriatric Medicine and Infectious Diseases, University of Michigan Medical School, Ann Arbor, and Veterans Affairs Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center (GRECC), Ann Arbor, MI.
References
1. Deafness and hearing impairment. Fact Sheet. World Health Organization. April 2010. http://www.who.int/mediacentre/factsheets/fs300/en/. Accessed August 31, 2010.
2. Newman CW, Sandridge SA. Hearing loss is often undiscovered, but screening is easy. Cleve Clin J Med 2004;71:225-232.
3. Cook JA, Hawkins DB. Hearing loss and hearing aid treatment options. Mayo Clin Proc 2006;81:234-237.
4. Bagai A, Thavendiranathan P, Detsky AS. Does this patient have hearing impairment? JAMA 2006;295:416-428.
5. Cheng YJ, Gregg EW, Saaddine JB, et al. Three decade change in the prevalence of hearing impairment and its association with diabetes in the United States. Pre Med 2009;49:360-364. Published Online: August 5, 2009.
6. Smeeth L, Fletcher AE, Ng ES, et al. Reduced hearing, ownership, and use of hearing aids in elderly people in the UK – The MRC Trial of the Assessment and Management of Older People in the Community: A cross-sectional survey. Lancet 2002;359:1466-1470.
7. Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: Audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 2008;149:1-10. Published Online: June 16, 2008.
8. Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: Contributing environmental and genetic factors. Audiol Neurol 2007;12:345-358. Published Online: July 27, 2007.
9. Dalton DS, Cruickshanks KJ, Klein BE, et al. The impact of hearing loss on quality of life in older adults. Gerontologist 2003;43:661-668.
10. Gates GA, Mills JH. Presbycusis. Lancet 2005;366:1111-1120.
11. Yueh B, Souza PE, McDowell JA, et al. Randomized trial of amplification strategies. Arch Otolaryngol Head Neck Surg 2001;127:1197-1204.
12. Hickson L, Worrall L, Scarinci N. A randomized controlled trial evaluating the active communication education program for older people with hearing impairment. Ear Hear 2007;28:212-230.
13. Yeuh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: Scientific review. JAMA 2003;289:1976-1985.
14. Yeuh B, Collins MP, Souza PE, et al. Screening for Auditory Impairment–Which Hearing Assessment Test (SAI-WHAT): RCT design and baseline characteristics. Contemp Clin Trials 2007;28:303-315. Published Online: August 30, 2006.
15. Popelka MM, Cruickshanks KJ, Wiley TL, et al. Low prevalence of hearing aid use among older adults with hearing loss: The Epidemiology of Hearing Loss Study. J Am Geriatr Soc 1998;46:1075-1078.
16. Chao TK, Chen TH. Cost-effectiveness of hearing aids in the hearing-impaired elderly: A probabilistic approach. Otol Neurotol 2008;29:776-783.
17. 1910.95: Occupational noise exposure. Occupational Safety & Health Administration. United States Department of Labor. http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9735. Accessed August 31, 2010.
18. Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: Data from the National Health and Nutrition Examination Survey, 1999-2002. Otol Neurotol 2009;30:139-145.
19. Cohen SM, Labadie RF, Haynes DS. Primary care approach to hearing loss: The hidden disability. Ear Nose Throat J 2005;84:26-31, 44.
20. Weinstein BE, Ventry IM. Audiometric correlates of the Hearing Handicap Inventory for the elderly. J Speech Hear Disord 1983;48:379-384.
21. Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA 1983;25:37-42.
22. Migirov L, Taitelbaum-Swead R, Drendel M, et al. Cochlear implantation in elderly patients: Surgical and audiological outcome. Gerontology 2010;56:123-128. Published Online: August 27, 2009.
23. Pirozzo S, Papinczak T, Glasziou P. Whispered voice test for screening for hearing impairment in adults and children: Systematic review. BMJ 2003;327:967.
24. Burkey JM, Lippy WH, Schuring AG, Rizer FM. Clinical utility of the 512-Hz Rinne tuning fork test. Am J Otol 1998;19:59-62.
25. Bielefeld EC, Tanaka C, Chen GD, Henderson D. Age-related hearing loss: Is it a preventable condition? Hear Res 2010;264:98-107. Published Online: September 6, 2009.
26. Connell SS, Balkany TJ. Cochlear implants. Clin Geriatr Med 2006;22:677-686.


