Irritable Bowel Syndrome in Children: Education, Reassurance, and Management
ABSTRACT: Irritable bowel syndrome (IBS), a common condition in children and adolescents, is characterized by chronic abdominal pain with associated changes in bowel habit. A positive diagnosis can be made clinically, and extensive or invasive investigation often is not required. The pathophysiology of pediatric IBS, as with that of adults, is not fully understood. Genetic and psychosocial risk factors have been identified, and accumulating evidence suggests roles for the gut microbiota, increased intestinal permeability, immune activation, and central nervous system dysfunction, which may be primary or secondary to gut derangements. The mainstays of management are education, reassurance, and a biopsychosocial approach. Specific therapies such as dietary changes, probiotics, peppermint oil, and other pharmacotherapy also may have a role in management.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) encountered in pediatric practice. It is characterized by abdominal pain or discomfort, an abnormal bowel habit linked to pain, and often bloating.1 By definition, there must be no evidence of anatomic, metabolic, or neoplastic processes that explain the symptoms.2 Inflammation once was considered to be an exclusion, but accumulating evidence points to the presence of subtle inflammation in subsets of patients with IBS.3 Clinically, IBS is categorized as either diarrhea-predominant (IBS-D) or constipation-predominant (IBS-C).
IBS occurs in approximately 8% to 12% of children4,5 and 5% to 17% of adolescents4,6,7 in population-based studies. Prevalence appears to increase with age.4,6
Various aspects of quality of life, including physical, emotional, social, and school functioning, are significantly lower in children with IBS compared with healthy controls and are comparable in children with well-defined organic gastrointestinal disease such as inflammatory bowel disease.8
A desirable approach to evaluation minimizes unnecessary and invasive investigations. No single therapy is effective, and current approaches focus on a biopsychosocial concept of illness. Evidence of persistence of functional gastrointestinal symptoms and impaired functioning into adulthood underlines the need to actively manage children with IBS.
ETIOLOGY
The causal pathways leading to IBS in children, as in adults, are not fully understood, although a growing body of evidence implicates numerous potentially interrelated factors.
Genetic and familial factors. The relative importance of genetic factors, shared physical and psychosocial environmental factors, and learned behavioral factors in IBS has not been fully delineated, although environment probably is the dominant factor. FGIDs are common in first-degree relatives, particularly the mothers, of children with IBS.9,10 With respect to IBS specifically, approximately one-third of children with IBS have a mother with IBS.11 Candidate gene studies have focused on polymorphisms in neurotransmitter pathways, notably the serotonin transporter; however, associations have not been replicated in large studies.12 Concordance rates for IBS in an adult twin study were 17.2% for monozygotic twins and 8.4% for dizygotic twins.13 The concordance between an affected dizygotic twin and his or her mother was similar to the monozygotic concordance rate, suggesting that social learning or other environmental factors might play an important role.
Psychological and behavioral factors. Comorbid psychological conditions seem to occur more commonly in children with FGIDs than in controls. Anxiety or depressive disorders were detected in almost 79% and 43%, respectively, of children with functional abdominal pain in one small study.14 Maternal anxiety or depressive illness is significantly more commonly associated with children with functional abdominal pain than with controls.10
If parents exhibit a reinforcing or solicitous response to their child’s IBS symptoms, the child views his or her own complaints with greater concern.9 Additionally, clinic visits for gastrointestinal complaints (and even for any reason) are more common in children of adults diagnosed with IBS than in a matched control group.15
Adverse life events occurring before the age of 18 years were significantly associated with IBS in a large retrospective case-control study of adults, with a stronger association noted for women.16 Despite the potential for recall bias, recall of sexual and emotional abuse within the IBS group was more strongly linked to IBS than was general trauma or physical punishment.
Infections, inflammation, and microbiota. Studies following persons with documented bacterial gastrointestinal infections have reached conflicting conclusions about the risk of developing IBS. In the short term (at least 6 months), there appears to be a higher incidence of IBS and other FGIDs following acute bacterial gastroenteritis when compared with controls.17 In the longer term, rates of IBS may not be higher than that of the general population; in one study, IBS symptoms were reported by only approximately 10% of survey respondents who had had documented Salmonella or Campylobacter enteritis 1 to 10 years earlier.18 The strongest evidence comes from a prospective observational study of children in Walkerton, Ontario, where the cumulative incidence of IBS over 8 years was significantly higher in those with reported or clinically suspected gastroenteritis following contamination of municipal water (10.5% vs 2.5%; odds ratio, 4.6; 95% confidence interval [CI], 1.6-13.3).19
Peripheral blood monocyte cells from children with IBS show reduced secretion of interleukin-10 (an anti-inflammatory cytokine) in response to bacterial lipopolysaccharide compared with those from controls, suggesting a possible role for dysregulation of gastrointestinal inflammation in IBS.20 Cow’s milk allergy in the first year of life has been associated with increased rates of IBS in childhood.21 Impaired gut barrier function also may play a direct or indirect role in the development of IBS, base on the finding of increased proximal gastrointestinal and colonic permeability in children with IBS compared with controls.22
Recent molecular genetic techniques have enabled rapid and detailed study of the composition of the gut microbiota. These studies show differences in relative quantities of various genera between children with IBS and controls.23,24 Furthermore, the subtype of IBS could be correctly predicted with 98.5% accuracy with a computer algorithm using microflora data from a small pediatric case-control study.24 Small intestinal bacterial overgrowth also has been suggested as contributing to IBS in some patients, with abnormal lactulose/methane breath tests more common in children with IBS than in controls; in particular, overgrowth is associated with bloating symptoms.25 It should be noted, however, that false-positive breath test results can occur in the setting of rapid intestinal transit.
Visceral hypersensitivity—gut and brain. Rectal sensory thresholds for pain assessed with barostat-balloon systems have been used to study the concept of hypersensitivity to a standardized stimulus in IBS. Children with IBS and other FGIDs have demonstrated lower rectal sensory thresholds for pain than controls and children with organic disease.26-28 One study found referral of pain to abnormal sites in children with IBS, suggesting abnormal sensory integration occurring centrally.29
Hyperresponsiveness to nongastrointestinal stimuli, measured using facial startle responses to auditory stimuli, was greater in children with IBS compared with controls, independent of comorbid anxiety.30
EVALUATION
The diagnosis of IBS can be simply and safely made clinically by taking a history and performing a thorough clinical examination. The Rome III diagnostic criteria define IBS in children as abdominal pain (or discomfort) occurring at least weekly for 2 months, accompanied by at least 2 of the following: improvement with defecation, change in frequency, and change in form of stools.2
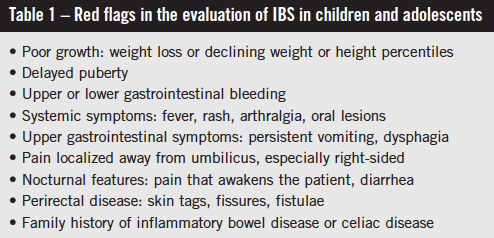
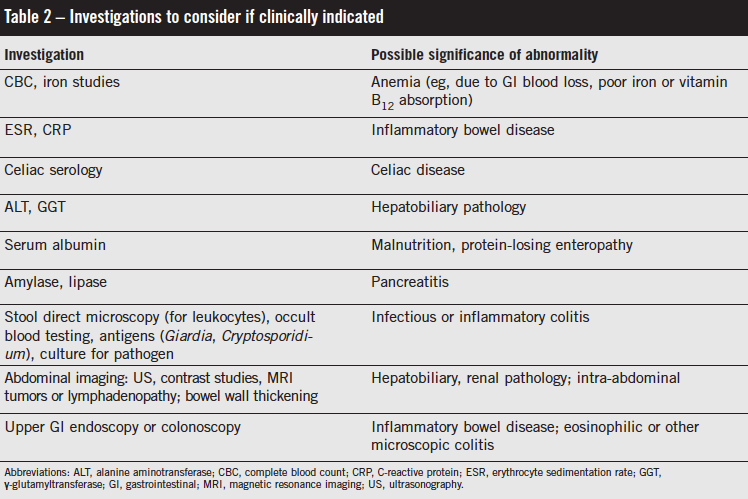
IBS is not a diagnosis of exclusion, and it does not usually require multiple investigations.1,14 Red flag symptoms often are suggested as being more associated with non-IBS causes (Table 1). The presence of these symptoms should prompt consideration of and possibly investigation for alternative organic etiologies. Nocturnal symptoms and abdominal tenderness on examination have been found to be more prevalent in children presenting with chronic or recurrent abdominal pain who were found to have organic disease.31 Where an alternative diagnosis is possible based on history and clinical examination, limited and focused investigations should be undertaken (Table 2).
Important elements of the history include the impact of symptoms on the physical, emotional, and academic functioning of the child. The parental response to illness and family history of FGIDs should be specifically determined. The presences of comorbid psychological illness such as anxiety or depression should be elicited, since this may affect symptoms and therapy. Early adverse life events or history of abuse also may be relevant.
CORE MANAGEMENT PRINCIPLES
Management of IBS in children involves making a positive diagnosis and educating and reassuring the child and caregivers about the condition. There also may be a role for therapies to treat specific symptoms, to avoid precipitating or aggravating factors, or to address implicated etiologic factors. These are summarized in Table 3.
It is important that treatment begin with an explanation to the child and his or her caregivers of what IBS is, and that it is a real condition with evidence of multiple contributing biologic factors. The benign nature of the condition should be discussed explicitly, since children and parents may be concerned about specific serious diseases such as malignancy or inflammatory bowel disease.
The physician must be empathetic and discuss realistic management goals, such as returning the child to previous levels of functioning, rather than promising complete resolution of symptoms. The chronic nature of IBS should be discussed, along with the likelihood of relapsing and remitting symptoms. Parental acceptance of a biopsychosocial model for recurrent abdominal pain is associated with symptom resolution in their children.32 Conversely, parental lack of insight about the influence of psychosocial factors on symptoms, as well as seeking opinions from multiple consultants, is associated with failure of improvement in children with functional abdominal pain.33 These same principles are likely to apply to IBS. When parents are coached to distract a child with FGID from their symptoms, the frequency of these complaints is reduced by at least half.34 The impact of parental response should be discussed explicitly.
General health measures such as ensuring a balanced diet, appropriate exercise, and sleep should be encouraged. Children with IBS may be on inappropriately restricted diets or may have developed unhealthy exercise or sleep habits. Specifically addressing these areas may improve functioning and the ability to cope with IBS symptoms. Obese children with FGIDs were more likely to have persistent and more debilitating symptoms on long-term follow-up.35 Thus, in addition to the general health benefits of weight reduction in obese and overweight children, weight management may assist with management of IBS in these children.
A discussion about the robust placebo response (up to 30%) in IBS in therapeutic trials36 should occur so that patients and parents may be better equipped to understand well-meaning friends and family members’ inevitable suggestions, which may be based on anecdotal experience. It also provides some context for discussing the evidence for some of the specific IBS therapies. The relationship between practitioner and patient seems to be the most influential factor in determining placebo response.36 Families also should be informed of the variable quality of information on the Internet about IBS and should be provided with reputable sources if required.

SPECIFIC MANAGEMENT OPTIONS
Diet. No specific diet has been demonstrated to be effective in managing IBS in a large number of patients with the condition. Time-limited trials of exclusion diets with appropriate consideration of potential nutritional deficiencies are safe and may be helpful. Because lactose intolerance may mimic IBS-D, a short (2-week) trial of a low-lactose diet may be helpful in these patients; however, it was not found to be effective as empirical therapy for children with recurrent abdominal pain.37,38 Little evidence supports an empirical gluten-free diet in children, although the diet did seem to help a subset of adults with IBS-D who had HLA-DQ2 or HLA-DQ8 alleles and positive celiac immunoglobulin G antibodies (but who did not meet histologic criteria for disease on duodenal biopsy).39 Diets low in FODMAPS (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), including fructose and fructans, can reduce symptoms in a subset of adults with IBS,40 but this diet has not been studied in children. Uncontrolled studies of children with functional abdominal pain and abnormal fructose-hydrogen breath tests suggest benefit of a low-fructose diet,41,42 but this has not been studied in IBS.
Patients with constipation may benefit from fiber supplementation, with psyllium husk having shown to be beneficial in adults with IBS-C43; however, the few studies undertaken in children and adolescents have not shown a benefit.44
Psychosocial interventions. These therapies are directed at assisting the child and parents to better cope with symptoms and enable the child to more effectively self-manage. Cognitive behavioral therapy (CBT) techniques involve much of the education aspects described above, in addition to equipping the child with an understanding of the impact of attitude about and behavior toward his or her condition on symptoms. Children are encouraged to monitor their pain, self-distract, and undertake relaxation exercises. Parents are taught to reinforce positive coping skills, direct the child to distraction, and not reinforce pain behavior with secondary gain. Randomized studies in children with recurrent abdominal pain demonstrate good efficacy45,46; however, details of the specific aspects of CBT often are not provided, making it difficult to reproduce these results in clinical practice.
Gut-directed hypnotherapy was superior to supportive therapy in recent reviews of randomized pediatric studies.47 Of note, 2 involved short-term (1-3 months) therapy with significant superiority to standard medical care that lasted beyond the treatment period and, in one of the studies, up to a year later. The cost of therapy was not reported; however, a third study involved listening to recorded hypnotherapy and guided imagery rather than a live therapist, and this was also superior to standard care. Longer-term follow-up (mean, 4.8 years; range, 3.4-6.7 years) of hypnotherapy-treated children showed sustained lower pain intensity and frequency scores, although functional impairment as measured by quality of life scores, doctors’ visits, and missed school or work days was no different than in the standard-care group.48
Altering gut microbiota: antibiotics and probiotics. While abnormal lactulose breath tests might be more prevalent in children with FGIDs, the few randomized studies of treatment have not shown benefit in treatment. Rifaximin for 10 days did not improve symptoms of chronic abdominal pain in one such placebo-controlled trial, with more than 90% of each group having abnormal baseline lactulose breath tests.49 Of note, only 20% of patients in the active treatment arm had normalized breath tests after therapy.
Probiotic therapy for IBS may be effective for some patients, but variability among organism strains, dosages, and duration make comparisons of studies difficult.50 Lactobacillus rhamnosus GG (LGG; 3 × 109 colony-forming units per day) for 8 weeks was superior in the short term to placebo in a double-blinded trial in 141 children with IBS or recurrent abdominal pain and seemed to be most effective in those with abnormal intestinal permeability tests.51 In a meta-analysis of 3 earlier trials involving 167 children with IBS, LGG supplementation was significantly associated with improved pain (number needed to treat = 4; 95% CI, 3-8).52 Note that most trials of probiotics have reported only short-term outcomes, and the placebo effect accounted for up to 50% of some outcome measures.53-55
Pharmacologic therapy. Medications such as antispasmodics, laxatives, and antidiarrheal agents have been used to treat specific IBS symptoms. Although evidence supports the use of antispasmodics as a class in adults with IBS,43 they do not have proven efficacy in children and may provoke anticholinergic effects. When using antidiarrheals or laxatives to treat IBS symptoms, keep in mind that while bowel habit may normalize, pain may not improve. This effect has been demonstrated in 2 adult IBS studies in which loperamide and polyethylene glycol 3350 had desirable effects on stool frequency in patients with IBS-D and IBS-C, respectively, but did not improve pain.56,57 Lubiprostone has a modest effect in adults with IBS-C,58 and linaclotide has been approved by the U.S. Food and Drug Administration for this indication, although published pediatric data are lacking.
Despite good evidence of efficacy in adults with IBS,59 randomized studies of amitriptyline have not consistently shown benefit for most outcomes in children with abdominal pain-related FGIDs.60 Amitriptyline was superior to placebo in a small double-blind study of adolescents with IBS, showing improvement in both pain and diarrhea where present,61 but a larger study reported no improvement in IBS symptoms at 4 weeks.62 Of note, however, the latter study had a very high placebo response rate (58%) compared with the active treatment arm (63%), and anxiety scores were reduced significantly in the amitriptyline group. Concern exists about the safety profile of these medications in children and adolescents with depression, which should prompt careful consideration of the risk-benefit ratio when considering their use. Thus amitriptyline may have a role, particularly for children and adolescents with severe IBS and significant coexisting anxiety. For other antidepressants, no evidence exists for efficacy in pediatric IBS.
Complementary and alternative medicine. Approximately 25% of children with FGIDs will have used complementary or alternative medicines for their condition,63 making it likely that the topic will come up in parents’ discussions. Among the most studied is peppermint oil, which may have smooth-muscle relaxing properties and has been found to be superior to placebo in randomized studies.64,65 One pediatric trial noted pain improvement in 76% of children at 2 weeks compared with 19% in the placebo group (P < .001) but no effect on bowel habit.65 Doses range from 1 to 2 capsules (180-200 mg each) three times daily, with heartburn and rectal discomfort/burning the main adverse effects.64
Acupuncture was superior to usual care66 or antispasmodic drugs, but not sham acupuncture in randomized, controlled studies of adults with IBS.67 No studies have been reported in children. Similarly, there are no published trials of homeopathy, naturopathy, chiropractic therapy, or spiritual healing in children with IBS.
PROGNOSIS
It is generally believed and often stated that most children with FGID improve with reassurance and time, and while this is supported by the generous placebo responses in therapeutic studies, there is little longitudinal data.33 Follow-up studies show higher rates of IBS symptoms, impaired functioning, and health care utilization than in controls.68 Anxiety and IBS may be more prevalent in young adults who experienced recurrent abdominal pain in childhood.69,70 The relationship appears to be independent of socioeconomic status, psychiatric illness, and maternal psychological distress.70 There is a stronger association between childhood chronic abdominal pain and adult IBS in individuals with a family history of IBS.71
Although evidence is lacking of a reduction in rates of adult IBS or FGID in children who were treated successfully for IBS, important evidence linking pediatric and adult FGIDs warrants efforts at successful management.72
REFERENCES:
1. Hyams JS. Irritable bowel syndrome, functional dyspepsia, and functional abdominal pain syndrome. Adolesc Med Clin. 2004;15(1):1-15.
2. Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527-1537.
3. Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010; 7(3):163-173.
4. Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996;129(2):220-226.
5. Dong L, Dingguo L, Xiaoxing X, Hanming L. An epidemiologic study of irritable bowel syndrome in adolescents and children in China: a school-based study. Pediatrics. 2005;116(3):e393-e396.
6. Sagawa T, Okamura S, Kakizaki S, Zhang Y, Morita K, Mori M. Functional gastrointestinal disorders in adolescents and quality of school life. J Gastroenterol Hepatol. 2013;28(2):285-290.
7. Devanarayana NM, Mettananda S, Liyanarachchi C, et al. Abdominal pain-predominant functional gastrointestinal diseases in children and adolescents: prevalence, symptomatology, and association with emotional stress. J Pediatr Gastroenterol Nutr. 2011;53(6):659-665.
8. Varni JW, Lane MM, Burwinkle TM, et al. Health-related quality of life in pediatric patients with irritable bowel syndrome: a comparative analysis. J Dev Behav Pediatr. 2006;27(6):451-458.
9. Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99(12):2442-2451.
10. Campo JV, Bridge J, Lucas A, et al. Physical and emotional health of mothers of youth with functional abdominal pain. Arch Pediatr Adolesc Med. 2007;161(2):131-137.
11. Buonavolontà R, Coccorullo P, Turco R, Boccia G, Greco L, Staiano A. Familial aggregation in children affected by functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2010;50(5):500-505.
12. Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology. 2010;138(4):1276-1285.
13. Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121(4):799-804.
14. Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113(4):817-824.
15. Levy RL, Whitehead WE, Von Korff MR, Feld AD. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000; 95(2):451-456.
16. Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10(4):385-390.
17. Saps M, Pensabene L, Di Martino L, et al. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008;152(6):812-816.
18. Schwille-Kiuntke J, Enck P, Zendler C, et al. Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil. 2011;23(11):e479-e488.
19. Thabane M, Simunovic M, Akhtar-Danesh N, et al. An outbreak of acute bacterial gastroenteritis is associated with an increased incidence of irritable bowel syndrome in children. Am J Gastroenterol. 2010;105(4):933-939.
20. Hua MC, Lai MW, Kuo ML, Yao TC, Huang JL, Chen SM. Decreased interleukin-10 secretion by peripheral blood mononuclear cells in children with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2011;52(4):376-381.
21. Saps M, Lu P, Bonilla S. Cow’s-milk allergy is a risk factor for the development of FGIDs in children. J Pediatr Gastroenterol Nutr. 2011;52(2):166-169.
22. Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 2008;153(5):646-650.
23. Rigsbee L, Agans R, Shankar V, et al. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2012;107(11):1740-1751.
24. Saulnier DM, Riehle K, Mistretta TA, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1782-1791.
25. Scarpellini E, Giorgio V, Gabrielli M, et al. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: a case-control study. J Pediatr. 2009;155(3):416-420.
26. Halac U, Noble A, Faure C. Rectal sensory threshold for pain is a diagnostic marker of irritable bowel syndrome and functional abdominal pain in children. J Pediatr. 2010;156(1):60-65.
27. Di Lorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr. 2001;139(6):838-843.
28. Van Ginkel R, Voskuijl WP, Benninga MA, Taminiau JA, Boeckxstaens GE. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology. 2001;120(1):31-38.
29. Faure C, Wieckowska A. Somatic referral of visceral sensations and rectal sensory threshold for pain in children with functional gastrointestinal disorders. J Pediatr. 2007;150(1):66-71.
30. Bakker MJ, Boer F, Benninga MA, Koelman JH, Tijssen MA. Increased auditory startle reflex in children with functional abdominal pain. J Pediatr. 2010;156(2):285-291.
31. El-Matary W, Spray C, Sandhu B. Irritable bowel syndrome: the commonest cause of recurrent abdominal pain in children. Eur J Pediatr. 2004; 163(10):584-588.
32. Crushell E, Rowland M, Doherty M, et al. Importance of parental conceptual model of illness in severe recurrent abdominal pain. Pediatrics. 2003;112(6 pt 1):1368-1372.
33. Lindley KJ, Glaser D, Milla PJ. Consumerism in healthcare can be detrimental to child health: lessons from children with functional abdominal pain. Arch Dis Child. 2005;90(4):335-337.
34. Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani TA. Parent attention versus distraction: impact on symptom complaints by children with and without chronic functional abdominal pain. Pain. 2006;122(1-2):43-52.
35. Bonilla S, Wang D, Saps M. Obesity predicts persistence of pain in children with functional gastrointestinal disorders. Int J Obes (Lond). 2011;35(4):517-521.
36. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999-1003.
37. Lebenthal E, Rossi TM, Nord KS, Branski D. Recurrent abdominal pain and lactose absorption in children. Pediatrics. 1981;67(6):828-832.
38. Dearlove J, Dearlove B, Pearl K, Primavesi R. Dietary lactose and the child with abdominal pain. Br Med J (Clin Res Ed). 1983;286(6382):1936.
39. Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5(7):844-850.
40. Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6(7):765-771.
41. Wintermeyer P, Baur M, Pilic D, Schmidt-Choudhury A, Zilbauer M, Wirth S. Fructose malabsorption in children with recurrent abdominal pain: positive effects of dietary treatment. Klin Padiatr. 2012;224(1):17-21.
42. Gomara RE, Halata MS, Newman LJ, et al. Fructose intolerance in children presenting with abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47(3):303-308.
43. Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313.
44. Horvath A, Dziechciarz P, Szajewska H. Systematic review of randomized controlled trials: fiber supplements for abdominal pain-related functional gastrointestinal disorders in childhood. Ann Nutr Metab. 2012;61(2):95-101.
45. Sanders MR, Rebgetz M, Morrison M, et al. Cognitive-behavioral treatment of recurrent nonspecific abdominal pain in children: an analysis of generalization, maintenance, and side effects. J Consult Clin Psychol. 1989;57(2):294-300.
46. Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol. 1994;62(2):306-314.
47. Rutten JM, Reitsma JB, Vlieger AM, Benninga MA. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch Dis Child. 2013;98(4):252-257.
48. Vlieger AM, Rutten JM, Govers AM, Frankenhuis C, Benninga MA. Long-term follow-up of gut-directed hypnotherapy vs. standard care in children with functional abdominal pain or irritable bowel syndrome. Am J Gastroenterol. 2012; 107(4):627-631.
49. Collins BS, Lin HC. Double-blind, placebo-controlled antibiotic treatment study of small intestinal bacterial overgrowth in children with chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2011;52(4):382-386.
50. Ringel Y, Ringel-Kulka T. The rationale and clinical effectiveness of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2011;45 suppl: S145-S148.
51. Francavilla R, Miniello V, Magistà AM, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126(6):e1445-1452.
52. Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33(12):1302-1310.
53. Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147(2):197-201.
54. Gawronska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25(2):177-184.
55. Guandalini S, Magazzu G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51(1):24-30.
56. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci. 1984;29(3):239-247.
57. Khoshoo V, Armstead C, Landry L. Effect of a laxative with and without tegaserod in adolescents with constipation predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23(1): 191-196.
58. Schey R, Rao SS. Lubiprostone for the treatment of adults with constipation and irritable bowel syndrome. Dig Dis Sci. 2011;56(6):1619-1625.
59.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
60. Kaminski A, Kamper A, Thaler K, Chapman A, Gartlehner G. Antidepressants for the treatment of abdominal pain-related functional gastrointestinal disorders in children and adolescents. Cochrane Database Syst Rev. 2011;(7):CD008013.
61. Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr. 2008;152(5):685-689.
62. Saps M, Youssef N, Miranda A, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology. 2009;137(4):1261-1269.
63. Vlieger AM, Blink M, Tromp E, Benninga MA. Use of complementary and alternative medicine by pediatric patients with functional and organic gastrointestinal diseases: results from a multicenter survey. Pediatrics. 2008;122(2):e446-e451.
64. Grigoleit HG, Grigoleit P. Peppermint oil in irritable bowel syndrome. Phytomedicine. 2005;12(8):601-606.
65. Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138(1):125-128.
66. MacPherson H, Tilbrook H, Bland JM, et al. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC Gastroenterol. 2012;12:150.
67. Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;(5):CD005111.
68. Walker LS, Guite JW, Duke M, Barnard JA, Greene JW. Recurrent abdominal pain: a potential precursor of irritable bowel syndrome in adolescents and young adults. J Pediatr. 1998;132(6):1010-1015.
69. Campo JV, Di Lorenzo C, Chiappetta L, et al. Adult outcomes of pediatric recurrent abdominal pain: do they just grow out of it? Pediatrics. 2001;108(1):E1.
70. Howell S, Poulton R, Talley NJ. The natural history of childhood abdominal pain and its association with adult irritable bowel syndrome: birth-cohort study. Am J Gastroenterol. 2005;100(9):2071-2078.
71. Pace F, Zuin G, Di Giacomo S, et al. Family history of irritable bowel syndrome is the major determinant of persistent abdominal complaints in young adults with a history of pediatric recurrent abdominal pain. World J Gastroenterol. 2006;12(24):3874-3877.
72. Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103(3):765-774


